Abstract
Aggregation-promoting factor (APF) was originally described as a protein involved in the conjugation and autoaggregation of Lactobacillus gasseri 4B2, whose corresponding apf gene was cloned and sequenced. In this report, we identified and sequenced an additional apf gene located in the region upstream of the previously published one. Inactivation of both apf genes was unsuccessful, indicating that APF function may be essential for the cell. Overproduction of APF proteins caused drastic alteration in the cell shape of this strain. These cells were irregular, twisted, enlarged, and tightly bound in unbreakable clumps of chains. Down-regulation of APF synthesis was achieved by cloning of the apf2 promoter region on a high-copy-number plasmid, which recruited a putative apf activator. As a consequence, the shape of the corresponding recombinant cells was elongated (filamentous) and cell division sites were no longer visible. None of the induced changes in APF production levels was clearly correlated with modifications of the aggregation phenotype. This report shows, for the first time, that APF proteins are mainly critical for L. gasseri 4B2 cell shape maintenance.
Lactic acid bacteria are normal inhabitants of the human oral cavity and gastrointestinal (GI) and urogenital tracts. Interest in the health-promoting effects of specific lactic acid bacterial strains has increased in recent years (10, 12). Survival and persistence of probiotic bacteria in the human GI tract is often reported to provide them a competitive advantage. One of the proposed mechanisms that could increase the potential of bacteria to survive and persist in the GI tract is their ability to aggregate (13). However, this hypothesis has never been confirmed.
To study the importance of aggregation, we chose an aggregating strain, Lactobacillus gasseri 4B2 (previously classified as L. plantarum 4B2), whose 32-kDa aggregation-promoting factor (APF) has already been described (25). This protein was purified from the culture supernatant as one of the most abundant proteins. The function of APF as a factor of aggregation was shown in vitro. After three subsequent washing steps, L. gasseri 4B2 lost its ability to aggregate. Addition of purified APF or of filtered supernatant reaggregated the washed cells. Furthermore, the role of APF in conjugation was also demonstrated (25). The frequency of pAMβ1 conjugal transfer (among Lactobacillus species whose aggregation is APF dependent) was increased upon addition of L. gasseri 4B2 filtered supernatant to the mating mixture. Since neither the apf gene nor any apf mutant was available, the exact physiological role of APF could not be demonstrated. Subsequent N-terminal sequencing of the L. gasseri 4B2 APF protein enabled the cloning and sequencing of the apf gene (accession no. Y08498; L. Morelli et al., direct submission).
Recently, we showed that four L. johnsonii and two L. gasseri strains contain two apf genes in their genomes (34). Analysis of the gene organization, amino acid composition, and physical properties of APF supported certain similarities of APF to S-layer proteins. Although APF was originally isolated from the cell supernatant, significant amounts of APF were found in a lithium chloride preparation of the surface proteins, showing that APF is able to noncovalently attach to the cell surface.
In this report, we demonstrate the presence of a second apf gene in L. gasseri 4B2. We further show that an imbalance of the APF concentration in the cell results in dramatic alterations of L. gasseri 4B2 cell shape.
MATERIALS AND METHODS
Bacterial strains and media.
L. gasseri strain 4B2 and its aggregation-negative mutant L. gasseri Agg−, which were used in the present study, were kindly provided by L. Morelli (University of Piacenza, Piacenza, Italy). Cloning in Escherichia coli was performed with strain EC101 (17). Lactobacillus strains were grown in Man-Rogosa-Sharpe (MRS) broth (Difco Laboratories, Detroit, Mich.) at 37°C for 24 h under static conditions or on MRS agar in an anaerobic atmosphere (with the AnaeroGen system [Oxoid, Basingstoke, United Kingdom]) supplemented with 10 μg of chloramphenicol per ml or 5 μg of erythromycin per ml where appropriate. E. coli was grown under agitation at 37°C in Luria-Bertani broth supplemented with 30 μg of chloramphenicol per ml or 150 μg of erythromycin per ml where appropriate.
Microbial techniques.
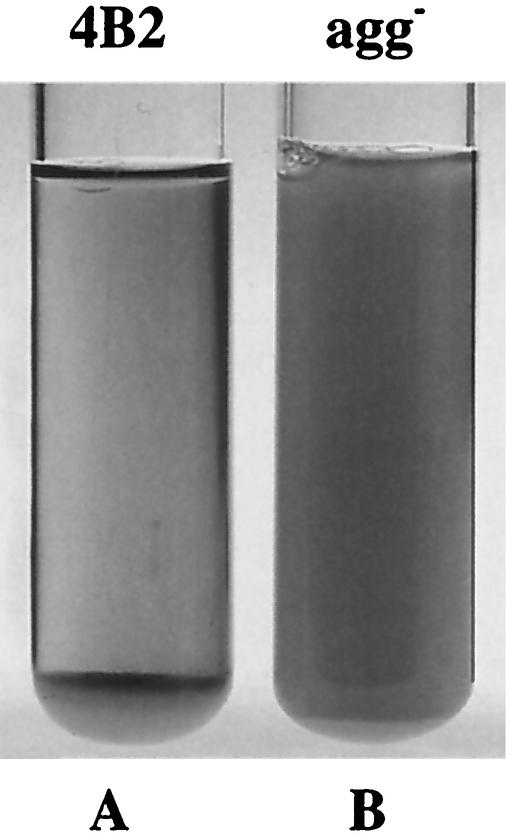
The aggregation phenotype was scored positive if the overnight cultures were clear with cells clumped at the bottom of the tube (Fig. 1). The strains were considered nonaggregating if the overnight cultures were turbid.
FIG. 1.
Aggregation and nonaggregation phenotypes. (A) Aggregating L. gasseri 4B2. (B) Nonaggregating L. gasseri Agg−.
The isogenicity of L. gasseri Agg− and 4B2 was confirmed by rapid amplified ribosomal DNA restriction analysis (33) with restriction enzymes Sau3AI, HinfI, and DraI and by the random amplified polymorphic DNA method (8).
Sensitivity of strains to different cell wall-targeted antibiotics was tested with ready-to-use antibiotic discs (BioMérieux). Bacterial cultures were plated on MRS plates, and antibiotic discs were placed on the agar surface. After 16 h of incubation at 37°C, the zones of inhibition were measured and compared to each other.
For plasmid stability testing, cultures were subcultured five times with no antibiotic. Each subculture was diluted and plated on MRS agar. The grown colonies were replicated on chloramphenicol (10 μg/ml)-containing MRS plates. The percentage of resistant colonies was calculated for each growth cycle.
DNA manipulations and transformation.
DNA manipulations, plasmid isolation, and transformation of E. coli were performed in accordance with standard procedures (26). All plasmid constructions were performed with E. coli. Plasmid DNA was introduced into Lactobacillus strains by electroporation (24). PCR was carried out with Taq polymerase (Roche) and the Expand high-fidelity PCR system (Roche).
Inactivation of apf genes of L. gasseri 4B2.
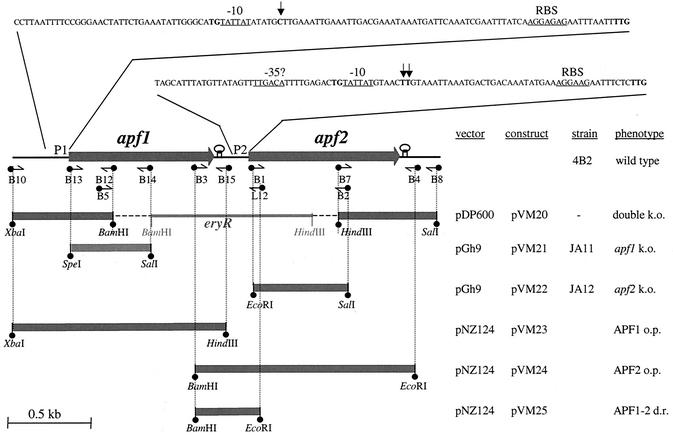
For double inactivation of the apf1 and apf2 genes, a derivative of plasmid pDP600 (R. D. Pridmore, unpublished data) was constructed. Plasmid pDP600 was constructed by exchanging the erythromycin resistance gene of pGh9 (19) with a chloramphenicol resistance gene. The erythromycin cassette (eryR) was reinserted back into the multiple cloning site, thereby creating multiple cloning sites upstream and downstream of the eryR gene. For double apf gene inactivation, the regions flanking apf1 and apf2 were cloned upstream and downstream of eryR, respectively. The upstream fragment was amplified with primers B10 and B12 (Table 1) and cloned into the XbaI and BamHI restriction sites of plasmid pDP600, upstream of the eryR cassette (Fig. 2). For downstream fragment amplification, primers B7 and B8 (Table 1) were used. The fragment was then cloned into the HindIII and SalI cloning sites downstream of the eryR cassette (Fig. 2). The resulting plasmid, pVM20, was transformed into L. gasseri 4B2, which was grown at a permissive temperature (30°C) in the presence of 10 μg of chloramphenicol per ml or 5 μg of erythromycin per ml. After raising the temperature to a nonpermissive 40°C, we screened for erythromycin-resistant, chloramphenicol-sensitive colonies in which gene replacement occurred and which had lost the plasmid.
TABLE 1.
Oligonucleotides used in this study
| Primer | Sequencea | Modification |
|---|---|---|
| B1 | 5′-AATCAGAATTCAATCTATCTTAACTAAG-3′ | EcoRI |
| B2 | 5′-AATCAGTCGACGTTGTACATTGTAGTTG-3′ | SalI |
| B3 | 5′-AATCAGGATCCCGCTACGGTTCATGGA-3′ | BamHI |
| B4 | 5′-AATCAGAATTCCACCAAAAAGGTGATCTTCC-3′ | EcoRI |
| B5 | 5′-CCTTGGTGCAAACCAATGGG | |
| B7 | 5′-AATCAAGCTTACAGCTACGCTTCAGC-3′ | HindIII |
| B8 | 5′-AATCAGTCGACGAACCAGCGCATGATGG | SalI |
| B10 | 5′-AATCATCTAGACTAAACGATGGGACGAGCG-3′ | XbaI |
| B12 | 5′-AATCAGGATCCAAGCAGTTGCGTGTGG-3′ | BamHI |
| B13 | 5′-AATCAACTAGTGCTTAACAATAATTGAAATCAAC-3′ | SpeI |
| B14 | 5′-AATCAGTCGACCTACTGAAGTCTTAGGAGCTG-3′ | SalI |
| B15 | 5′-AATCAAAGCTTAAAAGTGCCTTATGTTACAG-3′ | HindIII |
| L12 | 5′-AATCAGAATTCTACCAAACCAGTTGCGG | EcoRI |
| G1 | 5′-GTAAAACGACGGCCAGT-3′ | |
| N-L | 5′-GTCACTAACCTGCCCCG-3′ | |
| N-R | 5′-CTCATTGAGAAGATTGCCG-3′ | |
| A1 | 5′-CTTGTTGGCCAACAGTAACTTCTGC | IR800 |
| A2 | 5′-GCTACCAAACCAGTTGCGG-3′ | IR800 |
Introduced restriction site is underlined.
FIG. 2.
Genetic organization of the apf locus in L. gasseri 4B2. The nucleotide sequences of the apf1 and apf2 promoter regions are shown at the top. Putative RNA polymerase binding sites are underlined. Transcriptional start sites are shown in bold type and indicated by vertical arrows. Putative ribosomal binding sites (RBS) are underlined. Primers and their directions are represented by horizontal arrows. PCR amplicons are presented at the bottom, and cloning sites are indicated. The cloning strategy is outlined on the right. k.o., knockout; o.p., overproduction; d.r., down regulation.
To inactivate the apf1 gene of L. gasseri 4B2, a fragment proximal to the N terminus of the gene was amplified with primers B13 and B14 (Table 1; Fig. 2). The fragment was cloned into the SpeI and SalI sites of the pGh9 (19) temperature-sensitive vector to produce pVM21. For apf2 inactivation, primers B1 and B2 (Table 1; Fig. 2) were used. The amplified fragment was cloned into the EcoRI and SalI sites of the pGh9 vector to produce pVM22. The resulting plasmids, pVM21 and pVM22, were transformed into L. gasseri 4B2, which was grown at a permissive temperature (30°C) in the presence of 5 μg of erythromycin per ml. After the temperature was raised to a nonpermissive 40°C, erythromycin-resistant colonies containing the plasmid integrated into either of the apf genes in the chromosome were selected. Disruption of the apf1 gene was confirmed by PCR with primer B10 and pGh9-specific primer G1 (Table 1). Disruption of the apf2 gene was confirmed by PCR with primers B3 and G1 (Table 1).
Cloning of apf1 and apf2 into a high-copy-number plasmid.
The apf1 and apf2 genes of L. gasseri 4B2 were amplified from chromosomal DNA with the primer pairs B10/B15 and B3/B4, respectively (Table 1; Fig. 2). The apf1 fragment was cloned into the XbaI and HindIII restriction sites of pNZ124, and the apf2 fragment was cloned into the into BamHI and EcoRI restriction sites of pNZ124 (32). The resulting plasmids, pVM23 and pVM24, carried the apf1 and apf2 genes of L. gasseri 4B2 under the control of their own promoters, respectively. Correct cloning was confirmed by restriction analysis and DNA sequencing with pNZ124-specific primers N-L and N-R (Table 1).
Cloning of the apf2 promoter region into a high-copy-number plasmid.
The promoter region of the apf2 gene of L. gasseri 4B2 was amplified from chromosomal DNA with primers B3 and L12 (Table 1; Fig. 2). The amplicon was cloned into the BamHI and EcoRI restriction sites of pNZ124 (23) to produce pVM25. Correct cloning was confirmed by restriction analysis and DNA sequencing with pNZ124-specific primers N-L and N-R (Table 1).
DNA sequencing.
Sequences upstream of apf2 in L. gasseri 4B2 and the apf locus of L. gasseri Agg− were obtained by primer walking on chromosomal DNA or PCR fragments. All sequencing reactions were performed by Microsynth GmbH (Balgach, Switzerland).
RNA preparation and primer extension analysis.
For RNA isolation, the cells were harvested in the exponential growth phase. Bacterial cell pellets were disrupted with 106-μm glass beads in a Mini-Beadbeater-8 cell disrupter (Biospec Products). Total RNA was isolated with the RNeasy midi kit (Qiagen, Hilden, Germany). Primer extension analyses were performed as described previously (2), with avian myeloblastosis virus reverse transcriptase (Stratagene) and infrared dye (IRD800)-labeled primers. To map the transcription start sites of the apf1 and apf2 genes, primers A1 and A2 (Table 1), labeled with the infrared dye IRD800 at the 5′ end were used, respectively. Sequencing reactions were performed by the chain-terminating sequencing method (27) with a fluorescence-labeled primer cycle sequencing kit (Amersham Biosciences Europe GmbH, Freiburg, Germany) and the same primers. Reverse transcripts, together with sequencing reactions, were run on 8% polyacrylamide-urea gels and detected in a Li-Cor DNA sequencer.
Anti-APF antibodies.
Polyclonal anti-APF antibodies were raised in a rabbit (Eurogentec, Seraing, Belgium). Two polypeptides were selected from the APF sequence for a high probability of surface exposure and for a high antigenic index. The peptides EP011668 (H2N-CADNYVKSRYGSWTG-CONH2) and EP011669 (H2N-CGRESGGSYSARNGOY-CONH2) were synthesized and coupled with keyhole limpet hemocyanin. Testing of APF specificity by Western blot analysis showed that the polyclonal antibodies against the first peptide (EP011668) were more specific, and they were subsequently used in all experiments.
Western blot analysis.
Crude protein extracts were prepared from cultures grown overnight on MRS agar. The cells were scraped from the surface, washed in phosphate-buffered saline (PBS) (26), and disrupted in a Mini-Beadbeater-8 cell disrupter (Biospec Products). Protein concentrations were determined spectrophotometrically by the method of Bradford (5), and 20 μg of total protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto a nitrocellulose membrane. Western blot analysis was performed with polyclonal anti-APF antibodies and a chemiluminescent Western Breeze kit (Invitrogen).
Scanning electron microscopy.
The cells grown overnight were scraped from MRS agar, washed twice in PBS, fixed overnight with 2.5% glutaraldehyde in PBS, and washed with PBS. Postfixation was done with 1% osmium tetroxide in PBS, followed by dehydration in increasing concentrations of ethanol. Samples were subsequently dried to the critical point and coated with 20 nm of gold in a scanning electron microscopy coating unit (ES100, Polaron LTT). All samples were examined with a Philips SEM 505 scanning electron microscope at an accelerating voltage of 30 kV.
Nucleotide sequence accession number.
The DNA sequence reported here was submitted to the GenBank database and assigned accession no. AY245438.
RESULTS
apf sequence analysis.
In a previous study (25), only one APF protein of L. gasseri 4B2 was identified and the corresponding gene was sequenced later (accession no. Y08498). However, in all of the strains of L. gasseri and L. johnsonii analyzed so far, two adjacent apf genes have been found (34). To determine if another apf gene is present in the genome of L. gasseri 4B2, we sequenced the region upstream of the published apf gene, which, according to the sequence similarity, was a homologue of apf2. As expected, the presence of a second gene (apf1) upstream of apf2 was confirmed (Fig. 2).
Both apf genes are preceded by a putative ribosomal binding site and are initiated by a TTG start codon. Putative rho-independent terminator structures with calculated ΔG values of −10.8 and −13.2 kcal follow apf1 and apf2, respectively (Fig. 2). The corresponding APF1 and APF2 proteins are 261 and 297 amino acids long, with predicted molecular masses of 28.19 and 31.98 kDa, respectively. They exhibit 52% identity to each other on the amino acid sequence level. APF1 is preceded by a signal sequence with the predicted cleavage site VKA-AE between amino acids 38 and 39, while the predicted APF2 cleavage site, AQA-AT, lies between amino acids 34 and 35 (22).
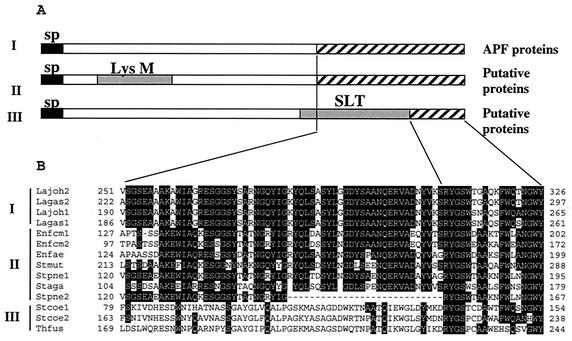
Similarity searches of databases identified several proteins sharing homology with the whole sequences of the APF proteins. The hypothetical proteins of the recently sequenced L. gasseri ATCC 33323 genome (accession no. ZP_00047488 and ZP_00047489) share 96 and 79% identity with L. gasseri 4B2 APF1 and APF2, respectively. The L. acidophilus APF protein (accession no. AJ438291) is 83% identical to L. gasseri 4B2 APF2. Moreover, proteins of five L. gasseri and L. johnsonii strains (34) show identities with L. gasseri 4B2 ranging from 48 to 99% for APF1 and from 81 to 99% for APF2. In addition, 10 hypothetical proteins sharing homology with the APF proteins only in the C-terminal part of the sequence were identified (Fig. 3). Closer sequence analysis revealed that these proteins, which are predicted to be secreted (22), could be classified into three different groups. The proteins of the first group consist of APF homologues for which surface localization has been demonstrated (34). The second group includes proteins containing LysM, a domain that, together with additional sites, probably mediates binding to the peptidoglycan (3). The proteins of the third group have a recognizable SLT motif, typical for lytic transglycosylases (16), which also need to be attached to the cell surface in order to reach their substrate. Thus, this C-terminal domain, shared by three different protein groups of different microorganisms, is a good candidate for a novel cell surface anchoring region in gram-positive bacteria.
FIG. 3.
(A) Domain architecture of proteins containing conserved C termini. Functionally distinct proteins containing a conserved C terminus were clustered into three groups: group I, containing APF proteins, group II, containing putative proteins with a LysM domain (peptidoglycan binding motif), and group III, containing putative proteins with an SLT domain (lytic transglycosylases). Signal peptides (sp) are indicated by black boxes. Conserved domains LysM and SLT are in gray. The homologous sequence region is indicated by striped boxes. (B) Alignment of conserved C termini. CLUSTAL software was used (11). Lagas1, APF1 protein from L. gasseri 4B2; Lagas2, APF2 protein from L. gasseri 4B2; Lajoh1, APF1 protein from L. johnsonii NCC533 (La1) (accession no. AAN63951); Lajoh2, APF2 protein from L. johnsonii NCC533 (La1) (accession no. AAN63952); Enfcm1, a protein of Enterococcus faecium (accession no. NZ_AAAK01000149); Enfcm2, a protein of E. faecium (accession no. NZ_AAAK01000213); Enfae, LysM domain protein of Enterococcus faecalis (EF0443, The Institute for Genomic Research, unfinished genome); Stpne1, LysM domain protein of Streptococcus pneumoniae (accession no. NC_003028); Stpne2, a protein of S. pneumoniae R6 (accession no. NC_003098); Stmut, LysM domain protein from Streptococcus mutans (accession no. NP_722430); Staga, LysM domain protein from Streptococcus agalactiae (accession no. NC_004116); Stcoe1, a protein from Streptomyces coelicolor (accession no. AL357591); Stcoe2, a protein from S. coelicolor (accession no. AL391754); Thfus, a protein from Thermobifida fusca (DOE_2021, Joint Genome Institute, unfinished genome).
Analysis of apf gene transcription.
To determine the transcriptional start points of apf1 and apf2, primer extension reactions were carried out with apf1- and apf2-specific primers. The transcriptional start point of the apf1 gene was determined to be at position 63 upstream of the apf1 coding region (Fig. 2). For apf2, two equally intense primer extension products were obtained at positions 42 and 43 upstream of the coding region (Fig. 2). Upstream of the transcription start sites, a −10 box (TATTAT) was identified in both promoter regions. While a typical −35 box was identified in the promoter region of apf2 at a suboptimal position only 11 bp upstream of the −10 region, no evident −35 box was found in the promoter region of apf1. The −10 hexamers of both apf promoters are extended by a TG motif at positions −13 and −14. The TG motif was shown to be responsible for the increased activity of the extended −10 boxes of some promoters with no recognizable −35 sequence (4, 6, 18). Thus, the apf1 and apf2 genes are independently transcribed from the promoters with TG extension of the −10 sequence and atypical −35 boxes.
Inactivation of apf genes.
To analyze the functional role of the L. gasseri APF proteins, we first tried to inactivate both the apf1 and apf2 genes. For that purpose, a double-crossover knockout construct, pVM20, was made (Fig. 2) and transformed into L. gasseri 4B2 and the inactivation procedure was performed as described in Materials and Methods. However, all attempts to obtain a double knockout were unsuccessful.
Inactivation of individual apf genes was achieved by a single plasmid integration. For inactivation of apf1 and apf2, pGh9 derivatives pVM21 and pVM22 were constructed (Fig. 2) and gene disruption was performed as described in Materials and Methods. Plasmid integration in the apf1 or apf2 gene by single crossover resulted in L. gasseri strains JA11 and JA12, respectively. The interruption of apf1 and apf2 was verified by PCR. The absence of APF1 or APF2 in the corresponding strain was confirmed by Western blot assays of crude cell extracts with anti-APF antibodies (Fig. 4, lanes 2 and 3). Notably, the overnight cultures of L. gasseri JA11 and JA12 with disrupted apf1 and apf2, respectively, were still clumping like the wild type, showing no changes in their ability to aggregate. Thus, single apf knockouts imply that coexistence of the two proteins is not required for cell aggregation and that only one protein is needed for survival.
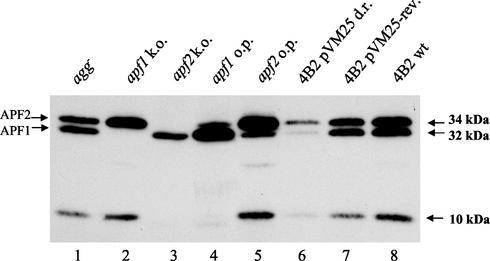
FIG. 4.
Western blot analysis of the APF proteins of L. gasseri Agg− (lane 1), JA11 (apf1 knockout; lane 2), JA12 (apf2 knockout; lane 3), 4B2(pVM23) (APF1 overproducer; lane 4), 4B2(pVM24) (APF2 overproducer; lane 5), 4B2(pVM25) (with APF down-regulated; lane 6), 4B2(pVM25) (wild-type-looking revertant; lane 7), and 4B2(pNZ124) (control; lane 8). The crude extracts from L. gasseri 4B2 cells grown on agar plates were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto a nitrocellulose membrane. The APF proteins were recognized with polyclonal anti-APF antibodies. k.o., knockout; o.p., overproduction; d.r., down regulation; rev., revertants; wt, wild type.
Characterization of the L. gasseri 4B2 aggregation-negative spontaneous mutant.
Since a double apf knockout seemed not to be viable in L. gasseri 4B2, we took the opportunity to analyze a spontaneous nonaggregating mutant of L. gasseri 4B2, L. gasseri Agg− (Fig. 1). This mutant was isolated after plating of a lyophilized L. gasseri 4B2 culture on MRS agar as a colony that appeared smoother and shinier than the wild type. This colony turned out to be a mutant that had lost the ability to aggregate in liquid medium. The isogenicity of L. gasseri Agg− and 4B2 was confirmed by rapid amplified ribosomal DNA restriction analysis and random amplified polymorphic DNA methods (data not shown).
We hypothesized that L. gasseri Agg− might be mutated in one of the apf genes, thus producing truncated or eventually no APF1 or APF2. We analyzed the APF proteins of L. gasseri Agg− by Western blotting. The amounts of APF proteins produced by the wild-type and mutant strain were similar (as estimated from several different blots [not shown]), and no truncation of APF1 or APF2 was observed in L. gasseri Agg− (Fig. 4, lanes 1 and 8). To exclude the possibility of small in-frame deletions or point mutations, we amplified the apf genes of L. gasseri Agg− with primers B10 and B4 and sequenced the amplified fragment. The sequence obtained from L. gasseri Agg− was 100% identical to the corresponding sequence in the wild-type strain. Despite the normal production of intact APF proteins, L. gasseri Agg− is not able to aggregate, suggesting that APF is not the sole component involved in aggregation and that another component(s) might be mutated in this strain.
APF overproduction in the wild-type and Agg− strains.
Since neither inactivation of the apf genes in the wild type nor complementation of the nonaggregating strain was possible, we analyzed the role of apf in cell metabolism by modulating the cellular concentrations of APF. To overproduce APF, the apf1 and apf2 genes were cloned with their own promoters into high-copy-number vector pNZ124 to produce pVM23 and pVM24, respectively (Fig. 2). The latter plasmids were transformed into wild-type L. gasseri 4B2, and overproduction of APF1 and APF2 in L. gasseri 4B2(pVM23) and 4B2(pVM24) was confirmed by Western blotting with anti-APF antibodies (Fig. 4, lanes 4 and 5). Upon overproduction of APF1, the amount of APF2 synthesized from the chromosomal gene copy was slightly reduced (Fig. 4, lanes 4 and 5). The same decrease in APF1 production was observed when APF2 was overproduced, suggesting the possibility of positive regulation of apf1 and apf2 gene expression. It should be noted that the 10-kDa band (Fig. 4) probably corresponds to the C-terminal part of the degraded APF2 protein and is recognized by C terminus-specific anti-APF antibodies.
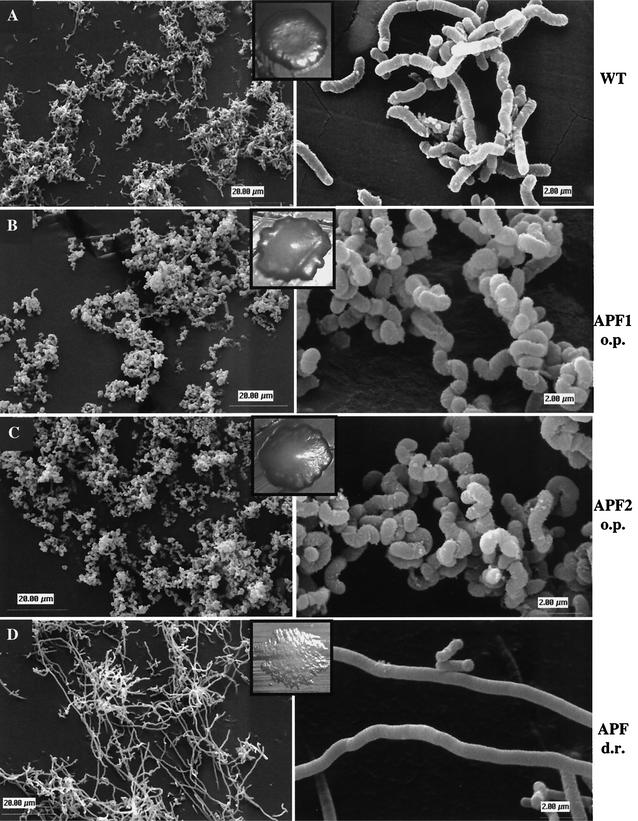
Careful observation of colony morphology revealed that the wild-type and APF-overproducing strains looked different on agar plates (Fig. 5). While wild-type colonies were flat, both APF-overproducing strains were convex. Microscopic analysis demonstrated that strains producing increased amounts of APF1 or APF2 had an altered cell shape (Fig. 5). They were twisted and exhibited an enlarged diameter compared to that of wild-type cells. Chains of the APF-overproducing strains were curled, producing tight clumps.
FIG. 5.
Scanning electron microscopic analysis of L. gasseri 4B2 strains. Cells grown on MRS agar were prepared as described in Materials and Methods. L. gasseri 4B2(pNZ124) (A) exhibits the wild-type morphology of rod-shaped cells; L. gasseri 4B2(pVM23) (B), overproducing APF1, and L. gasseri 4B2(pVM24) (C), overproducing APF2, exhibit twisted cells of increased diameter. L. gasseri 4B2(pVM25) (D), with downregulated APF production, exhibits elongated cells with no visible cell separation sites. Two different magnifications and the colony morphology are presented for each strain. WT, wild type; o.p., overproduction; d.r., down regulation.
In selective liquid medium, both APF1- and APF2-overproducing L. gasseri strains JA11 and JA12 grew as a mixed population of wild-type-looking cells and cells with altered shape (data not shown). Repeated attempts to isolate single colonies with a uniform phenotype failed. In the absence of selective pressure, L. gasseri 4B2 carrying pVM23 or pVM24 rapidly lost the plasmid and consequently reverted to wild-type morphology. After three subcultures, 90% of the cells were cured of the plasmid. This suggests that increased amounts of APF represent a metabolic burden for L. gasseri 4B2 cells.
When plasmids carrying apf1 or apf2 (pVM23 or pVM24, respectively) were transformed into L. gasseri Agg−, APF overproduction similar to that of the wild type was observed in Western blot assays (data not shown). The resulting strains, overproducing APF1 or APF2, were still nonaggregating but showed the same cell shape alterations as the APF-overproducing wild-type strains. Hence, the mechanisms by which APF influences cell shape and aggregation seem to be distinct.
APF down-regulation in the wild type.
While analyzing crude protein extracts of APF-overproducing strains L. gasseri 4B2(pVM23) and 4B2(pVM24), we noticed that overproduction of one APF protein led to a slight reduction in the level of the other APF protein (Fig. 4, lanes 4 and 5). This indicated that both apf genes may be regulated by the same positive regulator. In the APF1- and APF2-overproducing strains, this hypothetical activator may be recruited by the high copy number of apf from the plasmid (pVM23 or pVM24) and thereby be less available for activation of the chromosomal apf gene copies.
To test if apf transcription could be decreased by recruiting the hypothetical activator, we cloned the apf2 promoter region into high-copy-number plasmid pNZ124 to produce pVM25 (Fig. 2). Transformants of the wild-type strain exhibited a filamentous morphology (Fig. 5). Analysis of crude extracts by Western blot assays demonstrated that these cells produce much less APF1 and APF2 than the wild type (Fig. 4, lane 6), demonstrating that both apf genes are regulated by the same transcriptional activator. Microscopic analysis of the filamentous colonies revealed that the recombinant cells of L. gasseri 4B2 with down-regulated APF production were extremely elongated (Fig. 5). Such elongated cells had no obvious cell separation sites. This result confirmed the involvement of APF in the determination of L. gasseri 4B2 cell shape.
When grown in liquid medium, L. gasseri 4B2(pVM25) was still aggregating. However, microscopic analysis showed that the elongated cell phenotype was unstable even in the presence of antibiotic pressure. When the liquid culture of L. gasseri 4B2(pVM25) was plated on selective agar plates, two types of colony morphology appeared: filamentous and wild type looking. The spontaneous revertants exhibiting the wild-type cell shape still contained the intact pVM25 plasmid, but their levels of APF1 and APF2 were close to those of wild-type L. gasseri strain 4B2 (Fig. 4, lanes 7 and 8). This suggests that a suppression mutation occurred elsewhere in the genome, enabling optimal APF production. Thus, any imbalance of cellular APF concentrations definitely leads to cell shape alterations of L. gasseri 4B2.
DISCUSSION
In this study, we analyzed the physiological role of APF of L. gasseri 4B2. Of the six strains known to contain actively transcribed apf genes (34), three are nonaggregating (L. johnsonii NCC533 [La1] and ATCC 332 and L. gasseri ATCC 19992). Since the presence of apf genes was not obviously related to an aggregation phenotype, the role of APF in cell physiology deserved to be further studied. We first confirmed that L. gasseri 4B2 also contains two apf genes. All attempts to inactivate both apf genes failed. Single apf gene disruption was successful but did not change the ability of mutant L. gasseri strains JA11 and JA12 to aggregate. Moreover, sequencing of the spontaneous nonaggregating mutant form of L. gasseri 4B2, strain Agg−, revealed that its apf genes contained no mutations. The APF protein concentrations of L. gasseri Agg− were similar to those of the wild-type strain, suggesting that regulation of APF production was also intact. Since neither of the apf genes nor the level of APF production in L. gasseri Agg− was altered, it is possible that the mutation of L. gasseri Agg− lies in a second component involved in aggregation, e.g., an APF receptor molecule. In a previous study, it was shown that aggregation promoted by APF occurs only in strains containing α-1,2-glucose-substituted lipoteichoic or teichoic acid (25), which were proposed as APF receptors on the L. gasseri 4B2 cell surface. Lack of knowledge about APF receptors prevented us from identifying a suitable strain containing an APF receptor but carrying no apf genes in which heterologous complementation of aggregation could be shown. Actually, the results obtained in this study do not seem to support the role of APF proteins as aggregation factors. If APF proteins are indeed involved in cell aggregation, they probably need an additional actor(s) to be functional.
However, we clearly demonstrated that APF proteins influence the shape of L. gasseri 4B2 cells. As a consequence of APF overproduction, the cells of L. gasseri 4B2(pVM23) and 4B2(pVM24) became twisted and enlarged in diameter. A similar twisted cell shape phenotype was observed with mutants of penicillin binding protein PBP2a, which is involved in peptidoglycan production in Bacillus subtilis (28). Also, other mutations that influence peptidoglycan production cause different changes in the shape of B. subtilis (28) and E. coli (20, 21, 28, 30, 35) cells. Altered cell wall composition may lead to changes in the affinity of antibiotics for their target or their susceptibility to their target and make the cells more or less sensitive to cell wall-targeted antibiotics. However, we found no significant differences in the growth inhibition of the wild-type strain and strains containing increased APF copy numbers by penicillin G, vancomycin, cefotaxime, cefixime, or cefuroxime (data not shown). Thus, it seems unlikely that overproduction of APF influences L. gasseri 4B2 cell shape by interfering with peptidoglycan synthesis or regulation.
As shown by Western blot analysis of the crude extracts, when one APF protein was overproduced, the production of the other was reduced. In addition, an increased copy number of the apf2 promoter region caused the reduction of both APF protein levels in the cell, suggesting that both apf genes are regulated by an unknown positive regulator. The previous observation that apf genes are expressed more strongly in the exponential than in the stationary phase also indicated transcriptional apf regulation (34). Their strong expression in the exponential growth phase, together with the fact that the apf genes seem to be essential for cell survival, points to a role for APF in cell structure determination.
As a confirmation that APF influences cell shape in L. gasseri 4B2, the opposite cell shape alteration was obtained when APF production was reduced: the cells were elongated, with no visible cell division sites. Different variations of similar elongated cell phenotypes were observed with temperature-sensitive ftsZ, ftsQ, ftsA, and pbpB cell division mutant strains of E. coli (1, 31). The phenotype of the ftsZ mutant especially resembles that of the elongated cells of L. gasseri(pVM25). FtsZ is a homologue of the eucaryotic cytoskeleton protein tubulin, which is responsible for the initial phase of septum formation.
A comparison of the sequences of APF and FtsZ and other cell shape-affecting proteins revealed no significant matches. However, with the fold recognition technique 3D-PSSM (15), a tentative similarity with an E value of 1.07 was found with eucaryotic profilins, which are sophisticated regulators of polymerization of the main cytoskeleton protein actin (7). Recently, genes encoding analogues of eucaryotic actin, mreB or mlb, whose mutation resulted in cell shape alterations, were discovered in B. subtilis (14). Actin-like and Fts cell division proteins are conserved among bacteria, and the corresponding genes are present in the recently sequenced genome of L. gasseri (http://www.jgi.doe.gov/JGI_microbial/html/index.html). It could be hypothesized that APF interacts with these proteins or interferes with their synthesis and thereby influences the cell shape of L. gasseri 4B2. In gram-negative archaebacteria, it has been shown that the S-layer is involved in cell shape determination and in the cell division process (29). The amino acid composition, physical properties, and gene organization of APF indeed resemble those of S-layer proteins (34). However, whether APF can interact with cytoskeleton molecules and if the cell shape changes caused by APF are due to imbalance of cell division or actin-like molecules in L. gasseri 4B2 remains to be elucidated.
Genes affecting bacterial cell shape are typically essential for viability (9), which may explain our failure to obtain an apf double knockout. Double inactivation of apf genes was also unsuccessful in L. johnsonii NCC533 (La1) (34), suggesting that the physiological function of APF is essential. In addition, in the absence of selective pressure, both overexpressing (pVM23 and pVM24) and downregulating (pVM25) constructs were very unstable in L. gasseri 4B2. Moreover, the elongated cells of L. gasseri 4B2(pVM25) reverted spontaneously to the wild-type phenotype even in the presence of antibiotic pressure, which was accompanied by increased amounts of APF to wild-type levels. Thus, either by aborting the plasmid or by producing a suppression mutation, cells very quickly recover optimal cellular APF amounts.
Our data suggest, for the first time, that APF proteins are essential for L. gasseri 4B2, and they show that any changes in their cellular concentration lead to alteration of cell shape. Whether APF acts directly to control cell shape, indirectly interferes with processes like peptidoglycan, exopolysaccharide, lipoteichoic acid, and teichoic acid synthesis, or interacts with other proteins involved in bacterial cell shape maintenance remains to be established.
Acknowledgments
We thank L. Morelli (University of Piacenza, Piacenza, Italy) for providing L. gasseri strain 4B2 and its aggregation-negative mutant L. gasseri Agg− and for helpful suggestions. We are indebted to R. D. Pridmore for sharing unpublished results and for the gift of plasmid pDP600. We also thank Annick Mercenier, Jacques-Eduard Germond, Reinhold Brückner, and Harald Bruessow for discussions and critically reading the manuscript. We acknowledge Martin Grigorov for performing three-dimensional structure prediction of APF proteins.
REFERENCES
- 1.Addinall, S. G., E. Bi, and J. Lutkenhaus. 1996. FtsZ ring formation in fts mutants. J. Bacteriol. 178:3877-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassias, J., and R. Bruckner. 1998. Regulation of lactose utilization genes in Staphylococcus xylosus. J. Bacteriol. 180:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman, A., and M. Bycroft. 2000. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 299:1113-1119. [DOI] [PubMed] [Google Scholar]
- 4.Bidnenko, E., D. Ehrlich, and M. C. Chopin. 1995. Phage operon involved in sensitivity to the Lactococcus lactis abortive infection mechanism AbiD1. J. Bacteriol. 177:3824-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Burns, H., and S. Minchin. 1994. Thermal energy requirement for strand separation during transcription initiation: the effect of supercoiling and extended protein DNA contacts. Nucleic Acids Res. 22:3840-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson, L., L. E. Nystrom, I. Sundkvist, F. Markey, and U. Lindberg. 1977. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J. Mol. Biol. 115:465-483. [DOI] [PubMed] [Google Scholar]
- 8.Cocconcelli, P. S., M. G. Parisi, L. Senini, and V. Bottazzi. 1997. Use of RAPD and 16S rDNA sequencing for the study of Lactobacillus population dynamics in natural whey culture. Lett. Appl. Microbiol. 25:8-12. [DOI] [PubMed] [Google Scholar]
- 9.Costa, C. S., and D. N. Anton. 1993. Round-cell mutants of Salmonella typhimurium produced by transposition mutagenesis: lethality of rodA and mre mutations. Mol. Gen. Genet. 236:387-394. [DOI] [PubMed] [Google Scholar]
- 10.Dunne, C., L. Murphy, S. Flynn, L. O'Mahony, S. O'Halloran, M. Feeney, D. Morrissey, G. Thornton, G. Fitzgerald, C. Daly, B. Kiely, E. M. Quigley, G. C. O'Sullivan, F. Shanahan, and J. K. Collins. 1999. Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie Van Leeuwenhoek 76:279-292. [PubMed] [Google Scholar]
- 11.Higgins, D. G., A. J. Bleasby, and R. Fuchs. 1992. CLUSTAL V: improved software for multiple sequence alignment. Comput. Appl. Biosci. 8:189-191. [DOI] [PubMed] [Google Scholar]
- 12.Holzapfel, W. H., P. Haberer, J. Snel, U. Schillinger, and J. H. J. Huis in't Veld. 1998. Overview of gut flora and probiotics. Int. J. Food Microbiol. 41:85-101. [DOI] [PubMed] [Google Scholar]
- 13.Huis in't Veld, J. H. J., R. Havenaar, and P. Marteau. 1994. Establishing a scientific basis for probiotic R&D. Trends Biotechnol. 12:6-8. [DOI] [PubMed] [Google Scholar]
- 14.Jones, L. J., R. Carballido-Lopez, and J. Errington. 2001. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell 104:913-922. [DOI] [PubMed] [Google Scholar]
- 15.Kelley, L. A., R. M. MacCallum, and M. J. Sternberg. 2000. Enhanced genome annotation using structural profiles in the program 3D-PSSM. J. Mol. Biol. 299:499-520. [DOI] [PubMed] [Google Scholar]
- 16.Koonin, E. V., and K. E. Rudd. 1994. A conserved domain in putative bacterial and bacteriophage transglycosylases. Trends Biochem. Sci. 19:106-107. [DOI] [PubMed] [Google Scholar]
- 17.Law, J., G. Buist, A. Haandrikman, J. Kok, G. Venema, and K. Leenhouts. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, C. Q., M. L. Harvey, and N. W. Dunn. 1997. Cloning of a gene encoding nisin resistance from Lactococcus lactis subsp. lactis M189 which is transcribed from an extended −10 promoter. J. Gen. Appl. Microbiol. 43:67-73. [DOI] [PubMed] [Google Scholar]
- 19.Maguin, E., H. Prevost, S. D. Ehrlich, and A. Gruss. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J. Bacteriol. 178:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markiewicz, Z., J. K. Broome-Smith, U. Schwarz, and B. G. Spratt. 1982. Spherical E. coli due to elevated levels of d-alanine carboxypeptidase. Nature 297:702-704. [DOI] [PubMed] [Google Scholar]
- 21.Nelson, D. E., and K. D. Young. 2001. Contributions of PBP 5 and dd-carboxypeptidase penicillin binding proteins to maintenance of cell shape in Escherichia coli. J. Bacteriol. 183:3055-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 23.Platteeuw, C., G. Simons, and W. M. de Vos. 1994. Use of the Escherichia coli β-glucuronidase (gusA) gene as a reporter gene for analyzing promoters in lactic acid bacteria. Appl. Environ. Microbiol. 60:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raya, R. R., and T. R. Klaenhammer. 1992. High-frequency plasmid transduction by Lactobacillus gasseri bacteriophage φadh. Appl. Environ. Microbiol. 58:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reniero, R., P. Cocconcelli, V. Bottazzi, and L. Morelli. 1992. High frequency of conjugation in Lactobacillus mediated by an aggregation-promoting factor. J. Gen. Microbiol. 138:763-768. [Google Scholar]
- 26.Sambrook, J., and D. W. Russel. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shohayeb, M., and I. Chopra. 1987. Mutations affecting penicillin-binding proteins 2a, 2b and 3 in Bacillus subtilis alter cell shape and peptidoglycan metabolism. J. Gen. Microbiol. 133(Pt. 7):1733-1742. [DOI] [PubMed] [Google Scholar]
- 29.Sleytr, U. B., P. Messner, D. Pum, and M. Sara. 1993. Crystalline bacterial cell surface layers. Mol. Microbiol. 10:911-916. [DOI] [PubMed] [Google Scholar]
- 30.Spratt, B. G. 1975. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc. Natl. Acad. Sci. USA 72:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taschner, P. E., P. G. Huls, E. Pas, and C. L. Woldringh. 1988. Division behavior and shape changes in isogenic ftsZ, ftsQ, ftsA, pbpB, and ftsE cell division mutants of Escherichia coli during temperature shift experiments. J. Bacteriol. 170:1533-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Kranenburg, R., and W. M. de Vos. 1998. Characterization of multiple regions involved in replication and mobilization of plasmid pNZ4000 coding for exopolysaccharide production in Lactococcus lactis. J. Bacteriol. 180:5285-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ventura, M., I. A. Casas, L. Morelli, and M. L. Callegari. 2000. Rapid amplified ribosomal DNA restriction analysis (ARDRA) identification of Lactobacillus spp. isolated from fecal and vaginal samples. Syst. Appl. Microbiol. 23:504-509. [DOI] [PubMed] [Google Scholar]
- 34.Ventura, M., I. Jankovic, D. C. Walker, R. D. Pridmore, and R. Zink. 2002. Identification and characterization of novel surface proteins in Lactobacillus johnsonii and Lactobacillus gasseri. Appl. Environ. Microbiol. 68:6172-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wachi, M., M. Doi, S. Tamaki, W. Park, S. Nakajima-Iijima, and M. Matsuhashi. 1987. Mutant isolation and molecular cloning of mre genes, which determine cell shape, sensitivity to mecillinam, and amount of penicillin-binding proteins in Escherichia coli. J. Bacteriol. 169:4935-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]