Abstract
Previous studies have shown that the baboon and squirrel monkey are resistant to certain herpesviruses that cause serious infection or induce tumours in the cebus monkey and marmoset. This study was undertaken to evaluate the immunological competence of the four species of nonhuman primates. A variety of clinical immunological techniques were employed to evaluate the bursal, thymicdependent and phagocytic systems of immunity in these species. No primate was found to demonstrate immune competence of the same magnitude observed in normal humans.
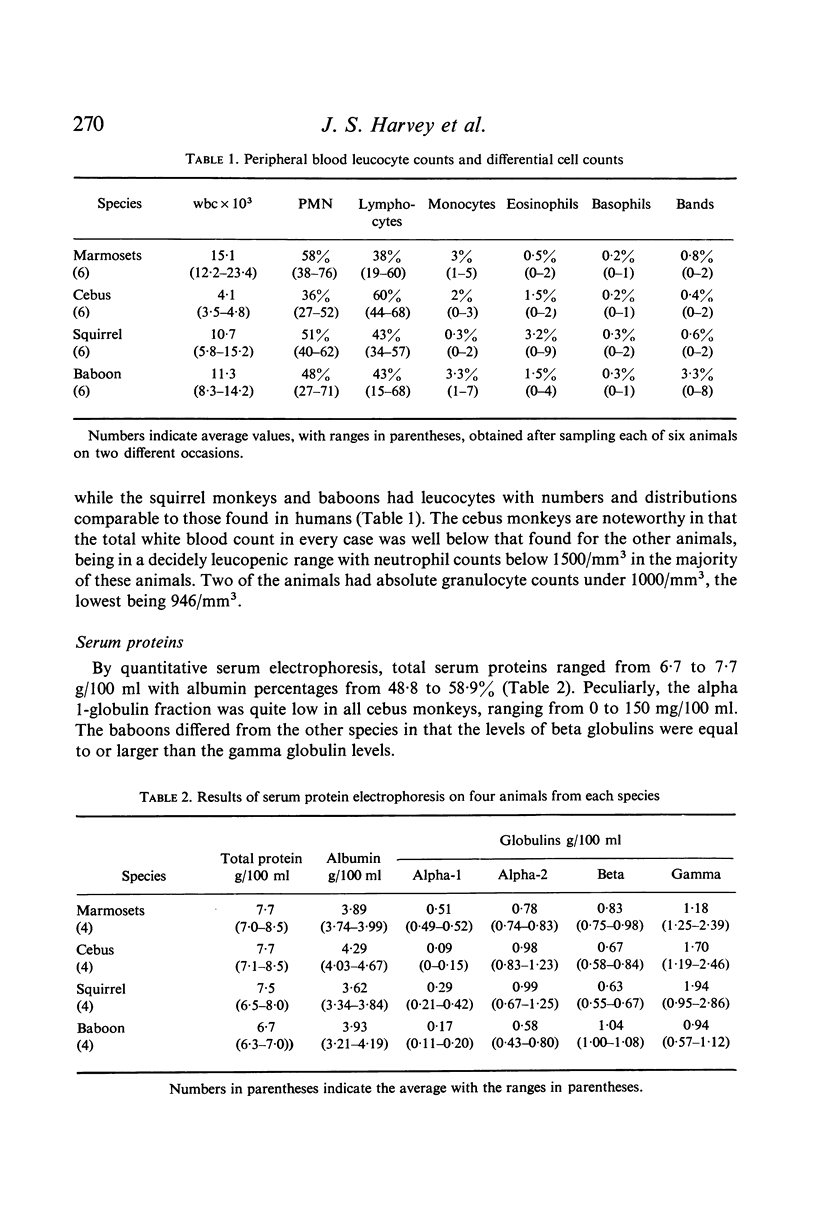
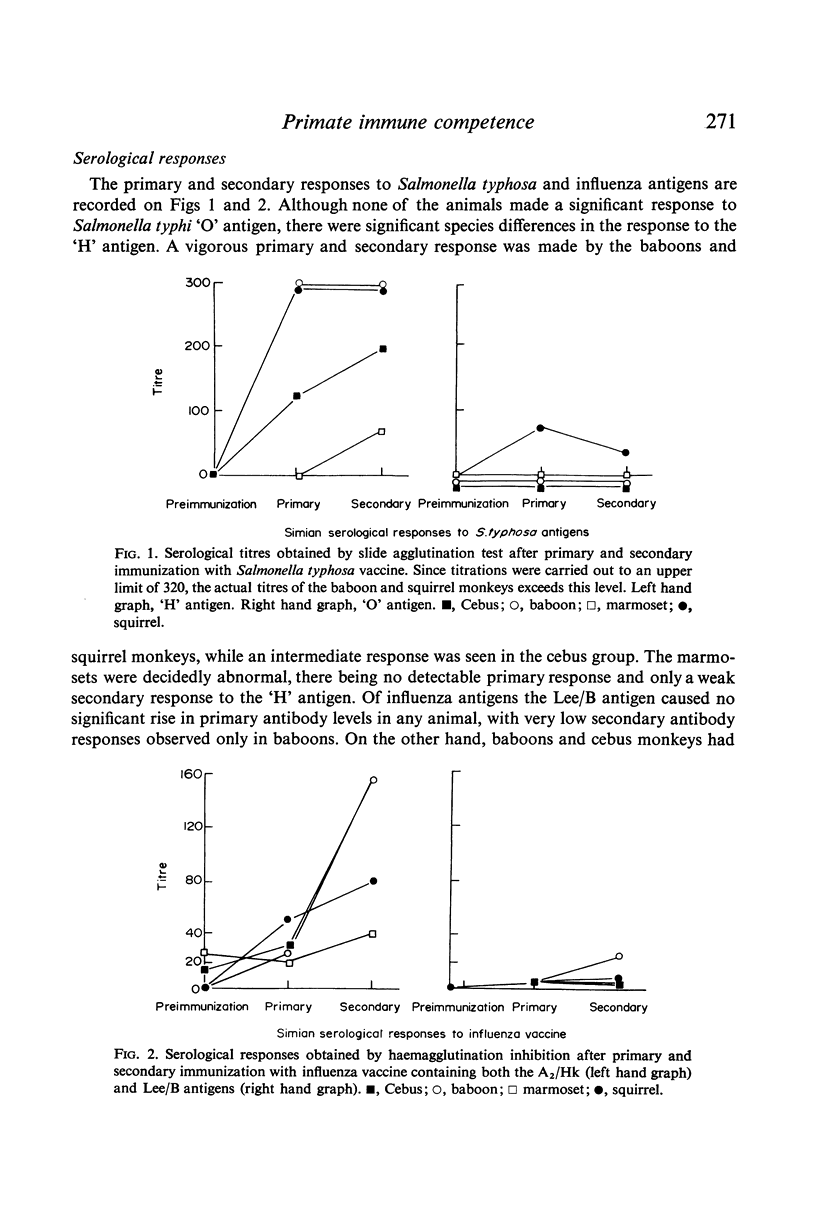
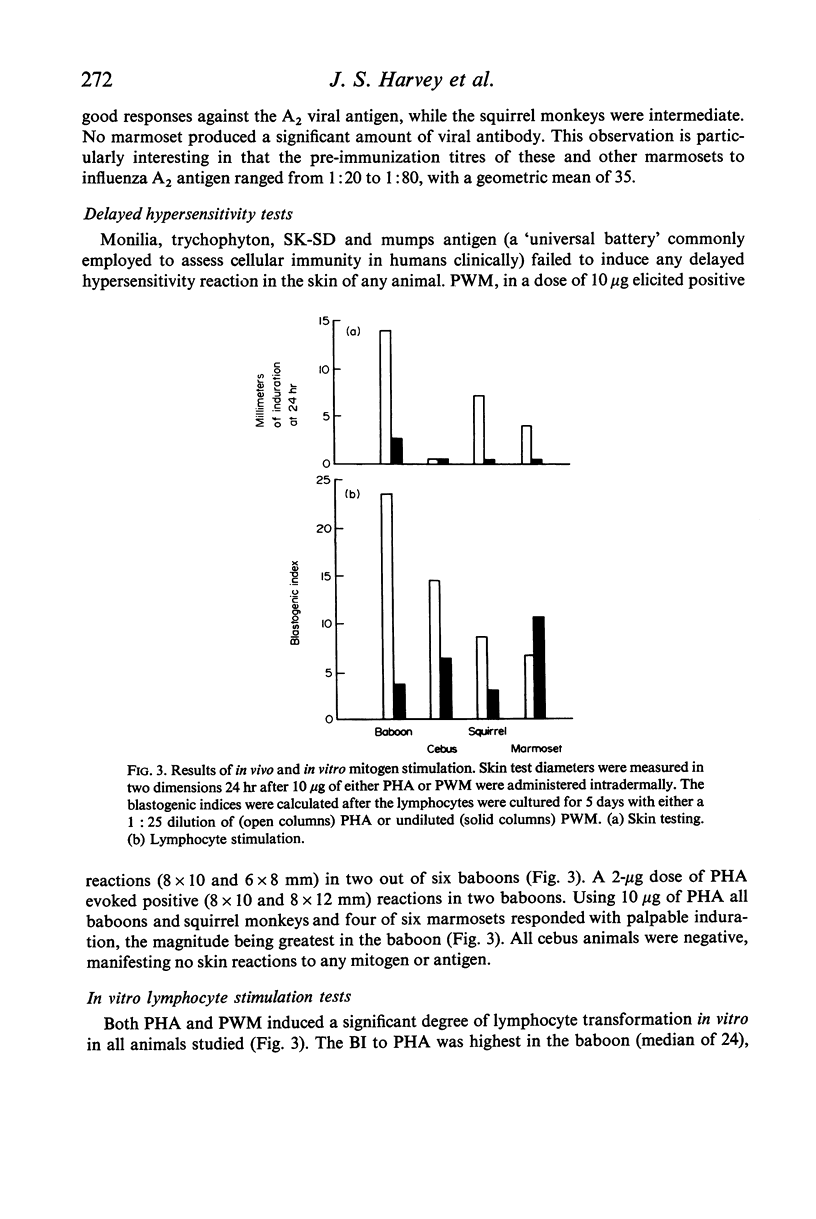
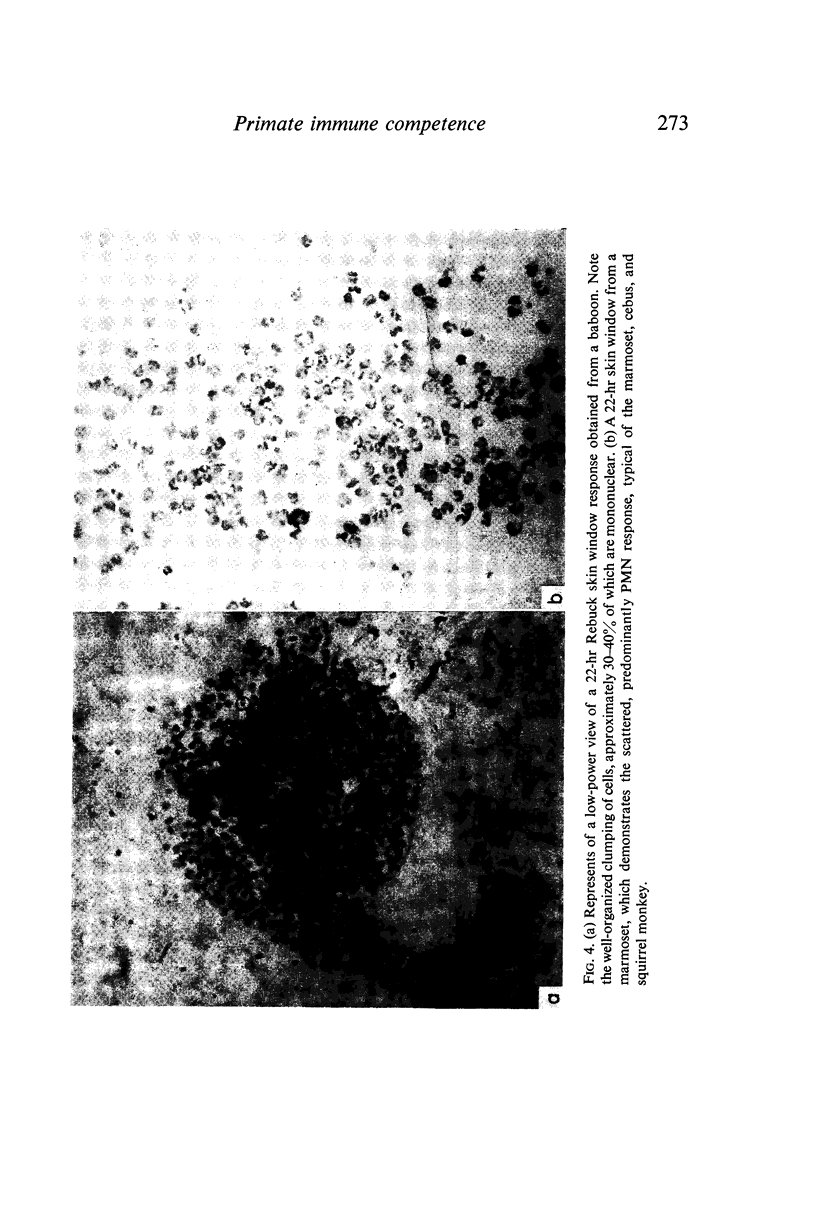
The baboon manifested immune responsiveness most closely resembling that of man. These animals displayed no abnormalities in circulating white blood cell populations, ability to form antibody, delayed skin response to mitogens or lymphocyte transformation in vitro. Rebuck skin window (RSW) findings closely resembled those observed in humans. While the squirrel monkey also appeared to be relatively competent, antibody formation, skin test reactivity and RSW responses were slightly less than those observed in the baboon. Cebus monkeys showed several abnormalities which included significant leukopenia, very low serum alpha-1 globulin levels, cutaneous anergy and a poor RSW response. The most marked abnormalities were found in the marmosets that had persistent leucocytosis, absent to poor antibody responses to immunogens, weak responses to phytohaemagglutinin in vivo and in vitro, with a uniquely greater response to pokeweed mitogen in vitro. The poor RSW response was similar to that observed in the squirrel monkey and cebus. While these studies suggest that baboons and squirrel monkeys are relatively intact immunologically, the marmoset and cebus monkey species appear to have significant manifestations of immunological incompetence in association with susceptibility to a variety of infectious and oncogenic agents.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonforte R. J., Topilsky M., Siltzbach L. E., Glade P. R. Phytohemagglutinin skin test: a possible in vivo measure of cell-mediated immunity. J Pediatr. 1972 Oct;81(4):775–780. doi: 10.1016/s0022-3476(72)80101-x. [DOI] [PubMed] [Google Scholar]
- Deinhardt F. Neoplasms induced by Rous sarcoma virus in New World monkeys. Nature. 1966 Apr 23;210(5034):443–443. doi: 10.1038/210443a0. [DOI] [PubMed] [Google Scholar]
- Deinhardt F., Wolfe L., Northrop R., Marczynska B., Ogden J., McDonald R., Falk L., Shramek G., Smith R., Deinhardt J. Induction of neoplasms by viruses in marmoset monkeys. J Med Primatol. 1972;1(1):29–50. doi: 10.1159/000460360. [DOI] [PubMed] [Google Scholar]
- Felsburg P. J., Heberling R. L., Kalter S. S. Experimental genital infection of cebus monkeys with oral and genital isolates of Herpesvirus hominis types 1 and 2. Arch Gesamte Virusforsch. 1972;39(1):223–227. doi: 10.1007/BF01241544. [DOI] [PubMed] [Google Scholar]
- Freyre E. A., Chabes A., Poémape O., Chabes A. Abnormal Rebuck skin window response in kwashiorkor. J Pediatr. 1973 Mar;82(3):523–526. doi: 10.1016/s0022-3476(73)80137-4. [DOI] [PubMed] [Google Scholar]
- Han T., Pauly J. Simplified whole blood method for evaluating in vitro lymphocyte reactivity of laboratory animals. Clin Exp Immunol. 1972 May;11(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Kalter S. S., Felsburg P. J., Heberling R. L., Nahmias A. J., Brack M. Experimental Herpesvirus hominis type 2 infection in nonhuman primates. Proc Soc Exp Biol Med. 1972 Mar;139(3):964–968. doi: 10.3181/00379727-139-36276. [DOI] [PubMed] [Google Scholar]
- MUNROE J. S., WINDLE W. F. Tumors induced in primates by chicken sarcoma virus. Science. 1963 Jun 28;140(3574):1415–1416. doi: 10.1126/science.140.3574.1415. [DOI] [PubMed] [Google Scholar]
- Meléndez L. V., Hunt R. D., Daniel M. D., Fraser C. E., García F. G., Williamson M. D. Lethal reticuloproliferative disease induced in Cebus albifrons monkeys by Herpesvirus saimiri. Int J Cancer. 1970 Nov 15;6(3):431–435. doi: 10.1002/ijc.2910060314. [DOI] [PubMed] [Google Scholar]
- Meléndez L. V., Hunt R. D., Daniel M. D., García F. G., Fraser C. E. Herpesvirus saimiri. II. Experimentally induced malignant lymphoma in primates. Lab Anim Care. 1969 Jun;19(3):378–386. [PubMed] [Google Scholar]
- REBUCK J. W., CROWLEY J. H. A method of studying leukocytic functions in vivo. Ann N Y Acad Sci. 1955 Mar 24;59(5):757–805. doi: 10.1111/j.1749-6632.1955.tb45983.x. [DOI] [PubMed] [Google Scholar]
- Sanford K. K., Jackson J. L., Parshad R., Gantt R. R. Evidence for an inhibiting influence of fetal bovine serum on "spontaneous" neoplastic transformation in vitro. J Natl Cancer Inst. 1972 Aug;49(2):513–518. [PubMed] [Google Scholar]
- Smith R. T. Possibilities and problems of immunologic intervention in cancer. N Engl J Med. 1972 Aug 31;287(9):439–450. doi: 10.1056/NEJM197208312870905. [DOI] [PubMed] [Google Scholar]
- Wolfe L. G., Falk L. A., Deinhardt F. Oncogenicity of herpesvirus saimiri in marmoset monkeys. J Natl Cancer Inst. 1971 Nov;47(5):1145–1162. [PubMed] [Google Scholar]
- Zilber L. A., Lapin B. A., Adgighytov F. I. Pathogenicity of Rous sarcoma virus for monkeys. Nature. 1965 Mar 13;205(976):1123–1124. doi: 10.1038/2051123a0. [DOI] [PubMed] [Google Scholar]