Abstract
Turnover studies were performed in rabbits using biologically screened, highly purified, radio-labelled human C3 and C3c. Experiments were also carried out using agents known to activate the complement system in vivo—cobra venom factor, human nephritic serum and nephrotoxic antibody to rabbit glomerular basement membrane. Activation of labelled C3 by cobra factor provided information regarding the metabolic behaviour of C3d.
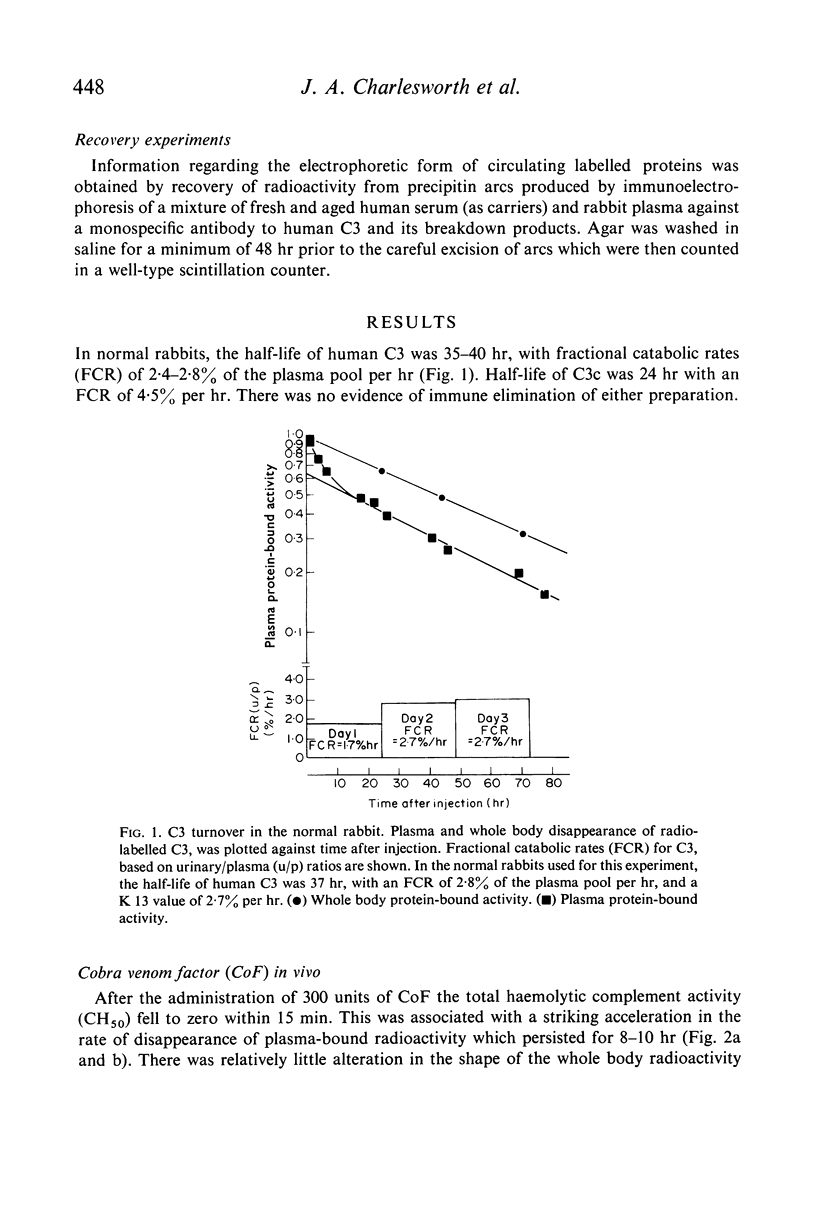
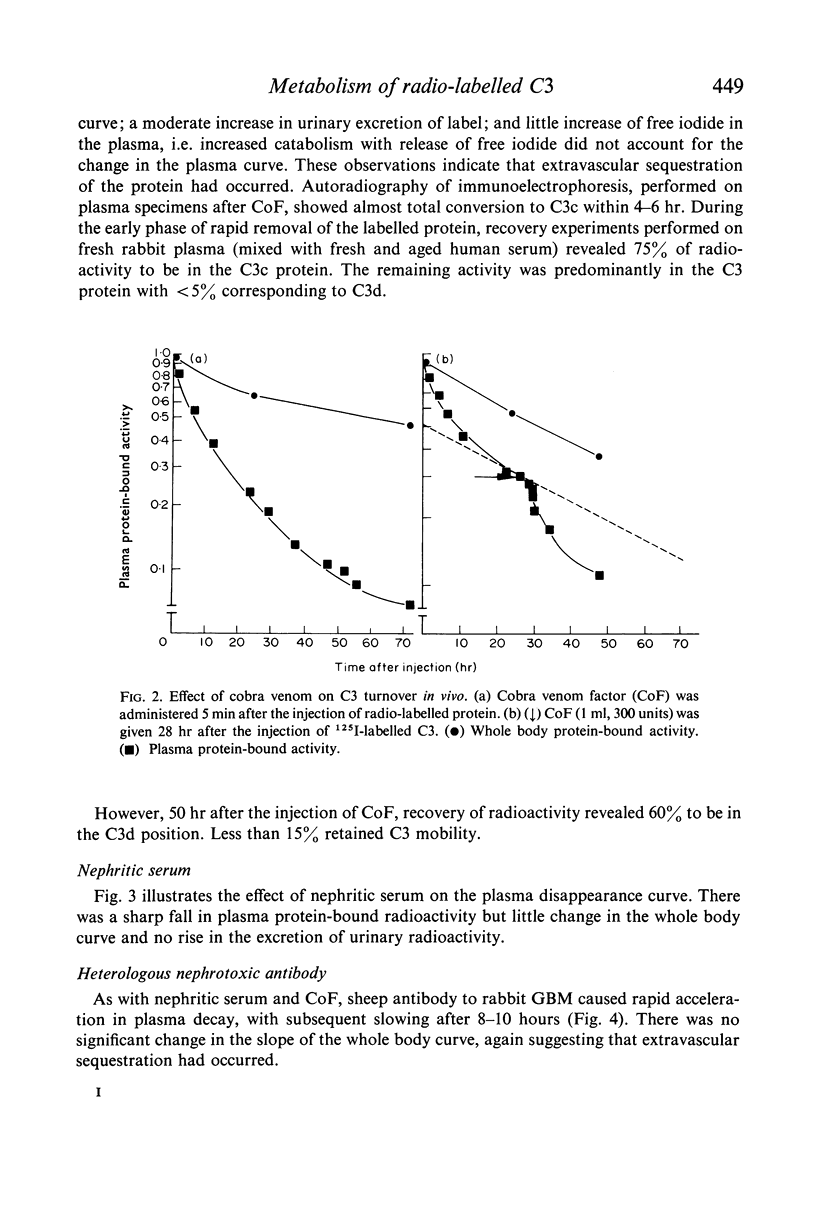
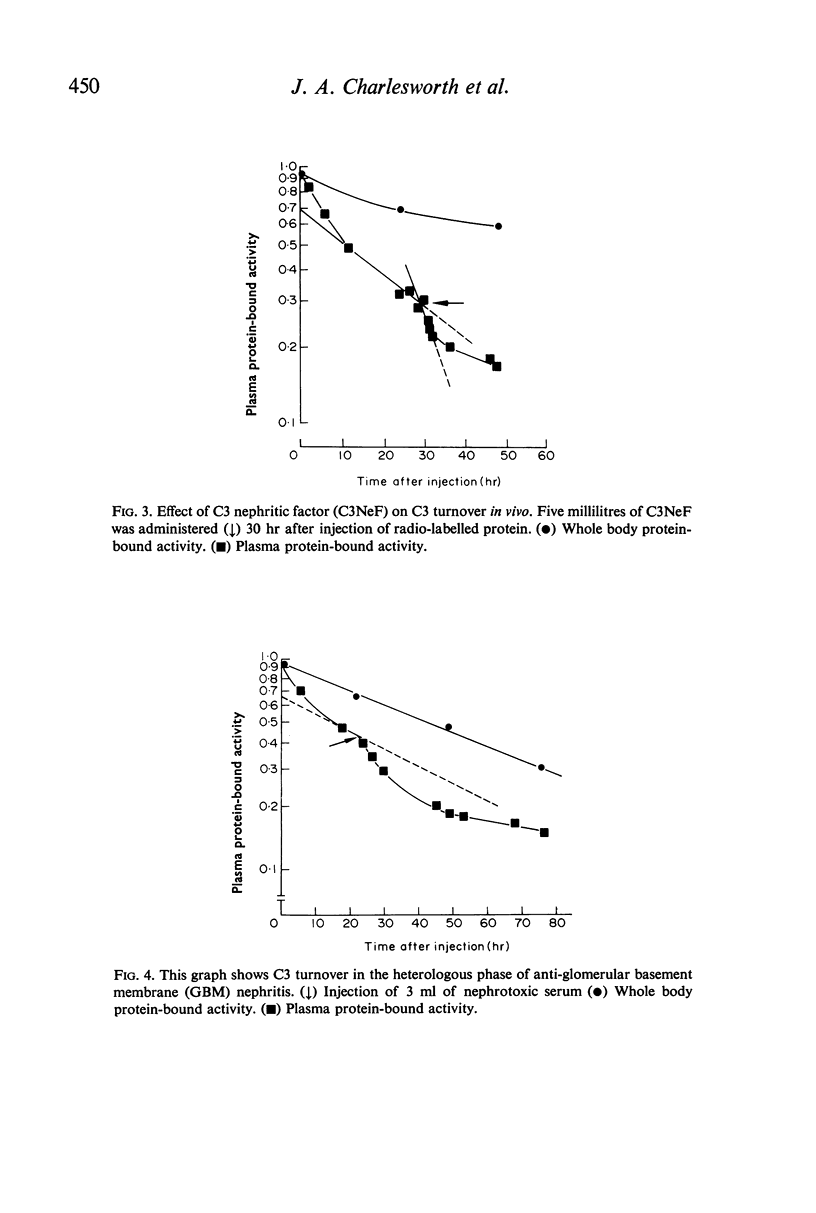
The fractional catabolic ratio (FCR) of human C3 in normal rabbits was 2·4–2·8% of the plasma pool per hr. FCR for C3c was 4.5% per hr. Activation of C3 in vivo consistently resulted in accelerated disappearance of plasma radioactivity. Analysis of the plasma and total body radioactivity curves indicated that both hypercatabolism and extravascular sequestration of radioactivity were responsible for this phenomenon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Rosen F. S. Alper CA, Rosen FS: Studies of the in vivo behavior of human C'3 in normal subjects and patients. J Clin Invest. 1967 Dec;46(12):2021–2034. doi: 10.1172/JCI105691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballow M., Cochrane C. G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969 Nov;103(5):944–952. [PubMed] [Google Scholar]
- Hunsicker L. G., Ruddy S., Carpenter C. B., Schur P. H., Merrill J. P., Müller-Eberhard H. J., Austen K. F. Metabolism of third complement component (C3) in nephritis. Involvement of the classic and alternate (properdin) pathways for complement activation. N Engl J Med. 1972 Oct 26;287(17):835–840. doi: 10.1056/NEJM197210262871701. [DOI] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Peters D. K., Martin A., Weinstein A., Cameron J. S., Barratt T. M., Ogg C. S., Lachmann P. J. Complement studies in membrano-proliferative glomerulonephritis. Clin Exp Immunol. 1972 Jul;11(3):311–320. [PMC free article] [PubMed] [Google Scholar]
- Pickering R. J., Herdman R. C., Michael A. F., Vernier R. L., Gewurz H., Fish A. J., Good R. A. Chronic glomerulonephritis associated with low serum complement activity (chronic hypocomplementemic glomerulonephritis). Medicine (Baltimore) 1970 May;49(3):207–226. doi: 10.1097/00005792-197005000-00002. [DOI] [PubMed] [Google Scholar]
- Spitzer R. E., Vallota E. H., Forristal J., Sudora E., Stitzel A., Davis N. C., West C. D. Serum C'3 lytic system in patients with glomerulonephritis. Science. 1969 Apr 25;164(3878):436–437. doi: 10.1126/science.164.3878.436. [DOI] [PubMed] [Google Scholar]
- Williams D. G., Lachmann P. J., Charlesworth J. A., Peters D. K. Role of C3b in the breakdown of C3 in hypocomplementaemic mesangiocapillary glomerulonephritis. Lancet. 1973 Mar 3;1(7801):447–449. doi: 10.1016/s0140-6736(73)91877-1. [DOI] [PubMed] [Google Scholar]


