Abstract
Objective:
Manufacturers of commercially available “therapeutic” magnets claim that these magnets cause physiologic thermal effects that promote tissue healing. We conducted this study to determine if skin or intramuscular temperatures differed among magnet, sham, and control treatments during 60 minutes of application to the quadriceps muscle.
Design and Setting:
A 3 × 3 mixed-model, factorial design with repeated measures on both independent variables was used. The first independent variable, application duration, had 3 random levels (20, 40, and 60 minutes). The second independent variable, treatment, had 3 fixed levels (magnet, sham, and control). The dependent variable was tissue temperature (°C). Measurement depth served as a control variable, with 2 levels: skin and 1 cm below the fat layer. Data were collected in a thermoneutral laboratory setting and analyzed using a repeated-measures analysis of variance.
Subjects:
The study included 13 healthy student volunteers (8 men, 5 women; age, 20.5 ± 0.9 years; height, 176.8 ± 10.4 cm; weight, 73.8 ± 11.8 kg; anterior thigh skinfold thickness, 16.9 ± 6.5 mm).
Measurements:
Temperatures were measured at 30-second intervals using surface and implantable thermocouples. Temperature data at 20, 40, and 60 minutes were used for analysis. Each subject received all 3 treatments on different days.
Results:
Neither skin nor intramuscular temperatures were different across the 3 treatments at any time. For both skin and intramuscular temperatures, a statistically significant but not clinically meaningful temperature increase (less than 1°C), was observed over time within treatments, but this increase was similar in all treatment groups.
Conclusions:
No meaningful thermal effect was observed with any treatment over time, and treatments did not differ from each other. We conclude that flexible therapeutic magnets were not effective for increasing skin or deep temperatures, contradicting one of the fundamental claims made by magnet distributors.
Keywords: intramuscular temperature, Hall voltage, biomagnetism
The use of magnetic fields to aid the body's healing response dates back to ancient Egypt and Greece.1,2 More recently, magnetic fields have been used in the treatment of musculoskeletal injuries,3–5 nerve dysfunction,6–9 pain,3,10–14 fractures,15–17 and osteoarthritis.18,19
Touting information from unpublished studies,5,20 magnet manufacturers and distributors have claimed that magnetic fields are associated with an increase in blood flow. The theory behind the use of magnetic fields to increase blood flow stems from the physics principle known as the Hall effect. The Hall effect is an electromotive force that causes charged particles to accumulate with like charges in the presence of a magnetic field.21,22
The charged particles in blood may, in the presence of a magnetic field, accumulate toward like poles. The magnetic field–induced voltage produced by this accumulation of charged particles against a concentration gradient is known as Hall voltage.22 The movement of these particles is resisted because the particles are forced to accumulate against their normal direction of flow. This migration against resistance may cause the production of heat that, in turn, would result in blood vessel dilation.
In addition to potential vasodilation, blood flow may be altered by another means. Pratt and Mishra,21 in work with saline solution and glass tubing, suggested that an ionic solution had greater flow in the presence of a magnetic field than in the absence of the field, independent of the diameter of the tube. In an effort to support their claim that magnetic fields caused vasodilation, magnet vendors reference the work of Pratt and Mishra,21 which was presented at a symposium but has not been subsequently published. To date, few investigators have examined whether magnetic fields alter blood flow in vivo.
Today, commercially available “therapeutic” magnets are essentially small sources of magnetic fields. Most are constructed of rubber impregnated with a magnetic material and induce low-level, static magnetic fields with a field strength of approximately 0.1 T (1000 G). Many allied health professionals, as well as many professional, collegiate, and recreational athletes, use these magnets. However, the usefulness of these magnets as a thermal modality able to produce clinically significant temperature changes has yet to be examined.
If magnets do, in fact, increase temperature and therefore blood flow, they may be effective as a thermal modality. Additionally, manufacturers claim that their products' magnetic fields have the ability to penetrate tissue more deeply and more safely when compared with other deep heating modalities such as ultrasound or diathermy.23 If these claims are accurate, there should be a measurable temperature change within the tissues during magnet application.
Although therapeutic magnets have been suggested to increase blood flow by increasing tissue temperature, this phenomenon has not been documented experimentally. The purpose of our study was to determine whether a temperature change occurs at the skin surface over the quadriceps muscle or at a depth of 1 cm below the adipose layer during a 60-minute application of magnet, sham, or control treatment. An additional purpose was to map the field strength and uniformity for a commercially available magnet.
METHODS
The experimental design of this study was a single-blind, 3 × 3 mixed-model factorial with repeated measures on both independent variables. The first independent variable, application duration, had 3 random levels (20, 40, and 60 minutes). The second independent variable, treatment, had 3 fixed levels (magnet, sham, and control). Measurement depth served as a control variable, with 2 levels: skin and 1 cm below the fat layer. The dependent variable was tissue temperature (°C).
Subjects
The subjects for this study were 13 university student volunteers (age, 20.5 ± 0.9 years; height, 176.8 ± 10.4 cm; weight, 73.8 ± 11.8 kg; thigh skinfold thickness, 16.9 ± 6.5 mm). Before participating, subjects were given a description of the study, signed an informed consent statement, and completed a health status questionnaire. Inclusion criteria consisted of a healthy right leg free from injury and infection and thigh skinfold-thickness measurement less than 40 mm. Approval of all procedures used in this study was obtained from the School's Human Subjects Committee.
Instrumentation
Temperatures were measured with implantable fine-wire thermocouples interfaced with an Iso-Thermex 16-channel electronic thermometer (Columbus Instruments, Columbus, OH). Type T, copper/constantan thermocouples (Model TX-31, Columbus Instruments) were used to measure thigh skin and ambient temperature of the room. Intratissue temperature was measured with implantable thermocouples (diameter, 0.4 mm) encased in a Teflon sheath (Model TX-23-21, Columbus Instruments). This equipment is accurate within ±1%24 and was recently calibrated.
Thigh skinfold-thickness measurements were made with calipers (Lafayette Instruments, Lafayette, IN) as described by Michael and Katch.25 Measurements for magnet field strength and uniformity were made with a gauss meter (Model 908, Magnetic Instruments, Indianapolis, IN). Statistical analyses were performed using SPSS for Windows (version 8.0, SPSS, Inc, Chicago, IL).
Testing Procedures
Tissue temperature measurements were made using a protocol previously described by Merrick et al.26 During each testing session, subjects assumed a supine position on a standard treatment table. A 2 × 2–cm area of the anterior thigh, midway between the patella and anterior superior iliac spine, was cleansed with an alcohol pad for 10 seconds. A sterilized, implantable thermocouple was inserted into a 21-gauge hypodermic needle until the tip of the thermocouple was visible but not protruding into the bevel of the needle.
The loaded hypodermic needle was inserted perpendicularly through the skin directly in the center of the cleansed area. The insertion depth for the thermocouple was 1 cm beyond the adipose layer. The adipose layer thickness was determined through a previously used method26 of halving the skinfold-thickness measurement.
The depth of insertion for each thermocouple was controlled using a mark made 5 cm from the tip of the thermocouple. The correct insertion depth was achieved by measuring the distance from the skin surface to these marks. Once the proper depth was achieved, the hypodermic needle was removed by pulling upward, along the thermocouple lead, leaving the thermocouple in place. Insertion depth was then remeasured, and lead wires were secured with Dermiclear tape (Johnson & Johnson, New Brunswick, NJ) directly over the insertion site and 2 cm distally. Latex gloves and universal precautions were used during insertion and extraction of the thermocouples. The skin-surface thermocouple was placed 1 cm distal to the inserted thermocouple and secured with Dermiclear tape.
Subjects did not receive any anesthesia before insertion of the thermocouple. Because flexible, fine-wire thermocouples were used rather than more rigid needle thermistors, subject discomfort was minimal. In fact, the only discomfort to the subject was from the initial needle stick. Once the needle was removed, most subjects reported no discomfort, and many stated that they were unable to feel the implanted thermocouple.
After thermocouple insertion, subjects lay supine for approximately 2 minutes to allow temperature to stabilize. At the end of those 2 minutes, a temperature reading was taken, and 1 of the 3 treatments (magnet, sham, or control) was applied. Immediately after treatment application, temperature measurements were taken at 30-second intervals for the duration of the treatment, a total of 60 minutes. At least 24 hours passed between subsequent experimental sessions.
A single 5 × 11–cm commercially available magnet (Nikken Inc, Los Angeles, CA) was used for all magnet treatments in this study in an effort to eliminate any between-magnet differences that might skew our results. This magnet was advertised as having field strength of 700 G (0.07 T) and properties capable of producing a low-level, homogeneous, direct-current static magnetic field. Before the study, the magnet's field strength and uniformity were measured using a gauss meter at arbitrarily selected points on the treatment surface. The meter reports field strength in gauss, and measurements of the field strength were made at points approximately 3 cm apart in a grid pattern over the side of the magnet to be applied to the patient. These measurements allowed for a comparison of advertised magnet strength with actual strength and also allowed some conclusion to be made about the uniformity of the field strength across the entire surface of the magnet.
The sham treatment was identical in size, thickness, and mass to the magnet but was made from silicone rubber and cork with no magnetic properties. Both the magnet and sham were covered with a checkerboard-patterned contact paper, making them identical in appearance. The magnet or sham was placed directly over the implanted and surface thermocouples and secured in place with pieces of Dermiclear tape. Control treatments consisted of temperature measurement only.
At the end of the 60-minute treatment, the thermocouple was removed, and a sterile adhesive bandage was applied to the insertion area. Subjects were given written instructions on wound care and infection and were scheduled for their next data-collection session as needed. After removal, the thermocouples were disinfected with Cidex Plus 2.4% glutaraldehyde solution (Johnson & Johnson Medical Inc, Arlington, TX).27 Hypodermic needles were used once and then discarded in an appropriate container for disposal.
Statistical Analysis
We performed a 3 × 3 factorial analysis of variance to determine if tissue temperatures differed across the treatments over time. Bonferroni post hoc analysis was used for simple main-effects pairwise comparison of interactions observed with the analysis of variance. The level of significance was set a priori at P ≤ .05.
RESULTS
Magnet Field Strength and Uniformity
From measurements made with a gauss meter at arbitrarily selected points, we found that the field strength of the magnet was quite variable and ranged from 6 G to 537 G, somewhat lower than the advertised field strength of 700 G. We could not calculate average field strength for the entire magnet. It was not feasible to measure field strength at every possible point on the magnet's surface because of the complex pattern of the magnetic elements within the magnet.
Skin and Intramuscular Temperatures
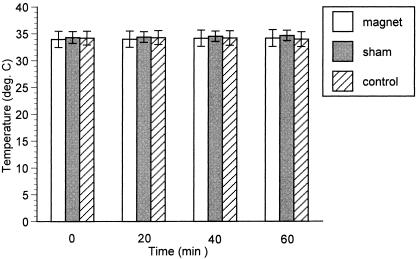
Ambient temperature during data collection averaged 22.3° ± 0.5°C. Skin and intramuscular temperatures for all treatments are reported in the Table. For intramuscular temperatures, a significant interaction was observed between treatment and time (F4,48 = 9.2, P < .001). Post hoc analysis revealed a difference for sham treatment between 20 and 60 minutes (P = .041) and between 40 and 60 minutes (P = .049). A difference was also observed for the control group between 20 and 60 minutes (P = .042). Although treatments did not differ from each other at any time, intramuscular temperatures had a tendency to rise very slightly for both the magnet and sham groups over the course of the treatment (Figure 1).
Skin and Intramuscular Temperatures for All Treatments*
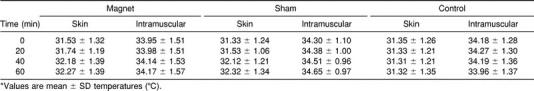
Figure 1.
Intramuscular temperatures did not differ among magnet, sham, and control treatments.
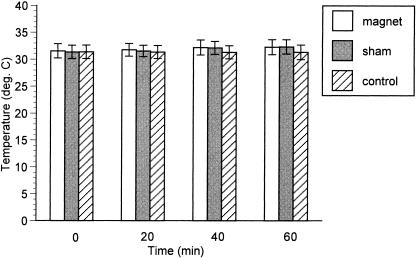
For skin temperatures, a significant interaction was also observed between treatment and time (F4,48 = 9.93, P < .001). Post hoc analysis revealed a difference for the magnet treatment between 20 and 60 minutes (P = .044), and for sham treatment, a difference between 20 and 40 minutes (P = .003) and 20 and 60 minutes (P = .012). Again, although no difference was observed among any of the treatments at any time, skin temperature also had a tendency to rise slightly for the magnet and sham groups over time (Figure 2).
Figure 2.
Skin temperatures did not differ among magnet, sham, and control treatments.
DISCUSSION
The physics phenomenon that magnet manufacturers commonly use to explain the usefulness of their product is the Hall effect. In their advertising materials, manufacturers make an unsupported leap from the Hall effect to vasodilation by suggesting that a Hall voltage is produced in the vasculature and that this voltage results in an increase in tissue temperature and a concomitant increase in blood flow.20–22,28 They suggest that the thermal effects produced by therapeutic magnets would be similar to other deep-acting thermal modalities, such as ultrasound or diathermy.29–32 They also suggest that the increase in perfusion supposedly produced by the magnets can be used to aid tissue repair, promote circulation, and reduce pain.29–32 The Food and Drug Administration has yet to approve magnet therapy, although it is currently under investigation by the National Institutes of Health.33,34 To date, the Food and Drug Administration has only restricted the use of magnetic fields that exceed 1000 G.3 As a result, magnet manufacturers have been able to advertise their products as a treatment for virtually any musculoskeletal disorder.3 Today many professional athletes33,35,36 and celebrities are strong supporters of magnetic therapy. Their support has influenced the spending of more than $500 million on magnet products in 1997 alone.33
In addition to advertising with celebrity and athlete endorsements, magnet vendors also commonly distribute advertising pamphlets,5,20 which contain the results of unpublished “medical studies” that support the use of their products. In these “studies,” magnet vendors make claims of increased circulation and temperature changes up to 3°C within 30 minutes of application of therapeutic magnets. Yet, an examination of the peer-reviewed literature regarding temperature changes with magnets reveals only 1 paper. Tenforde37 observed no thermal effect in rodents exposed to a high-intensity stationary magnetic field of 7.55 T. The magnetic field used in Tenforde's paper had a field strength 75 times the strength of most commercial therapeutic magnets, which typically have advertised field strengths of less than 0.1 T (1000 G).
In no prior studies have researchers examined the thermal effects of commercial therapeutic magnets. If the claims of the magnet manufacturers and vendors are correct, we should have observed an increase in tissue temperature during application of the magnet, and this increase should have been greater than any temperature increase observed during the sham or control conditions. We observed no such difference between treatment conditions. Although temperature did increase very slightly during both the 60-minute sham and magnet treatments, the increases were similar across the treatments, very small in magnitude (intramuscular increase of approximately 0.2°C, skin increase of approximately 0.8°C), and can most likely be explained by the insulating property of the magnet or sham preventing some small degree of heat loss to the environment during the treatment. It is unlikely that this temperature increase was the result of inflammation from the thermocouple insertion because it was not observed in the control group. From our results, we conclude that the application of therapeutic magnets did not increase either skin or intramuscular temperature when compared with a sham or control. Because no temperature effects were observed, claims based on these effects, such as vasodilation and increased blood flow, are at best unlikely, particularly considering that the field strength of these magnets may not be as great as advertised.
Our lack of an observable effect with therapeutic magnets is not unique. Borsa and Liggett3 examined the effect of magnets on pain perception, range of motion, and muscle strength after muscle microinjury. They observed no significant therapeutic effect on any of the variables studied.
The ability of therapeutic magnets to effectively increase tissue temperature is not supported by our findings. In fact, our results are in direct opposition to a fundamental claim of magnet manufacturers. It should be noted, however, that the magnet we used had a measured field strength that was considerably less than advertised and that the field strength was not uniform across the surface of the magnet. Had the magnet's field strength actually been 700 G, as advertised, it is possible, although unlikely, that our results might have been different. The less-than-advertised field strength of the magnet is somewhat troubling. Although it is probably not appropriate to draw meaningful conclusions about the accuracy of magnet strength claims based upon our experience with a single magnet, our study does provide some grounds for questioning these claims.
CONCLUSIONS
In this study, we attempted to document thermal effects of therapeutic magnets both at the skin surface and at 1 cm subadipose in healthy human thighs during 60 minutes of magnet application. We observed no difference in temperature among magnet, sham, and control treatments at any point during the treatments. We conclude that flexible therapeutic magnets did not affect tissue temperatures, that manufacturers' claims regarding thermal effects are unwarranted, and that any claims based directly on these presumed thermal effects are at best dubious. Additionally, some concern is raised regarding the actual versus advertised field strength of these magnets.
REFERENCES
- 1.Bauman A. Can magnets relieve pain? Runner's World. 1998;33(8):24. [Google Scholar]
- 2.Ruibal S. Ironclad cures for pain? Athletes put their faith in powers of magnets. USA Today. 1997 Aug 20;:C3. [Google Scholar]
- 3.Borsa PA, Liggett CL. Flexible magnets are not effective in decreasing pain perception and recovery time after muscle microinjury. J Athl Train. 1998;33:150–155. [PMC free article] [PubMed] [Google Scholar]
- 4.Railton R, Newman P. Magnetic field therapy—does it affect soft tissue? J Orthop Sports Phys Ther. 1983;4:241–246. doi: 10.2519/jospt.1983.4.4.241. [DOI] [PubMed] [Google Scholar]
- 5.Kokoschinegg P. Oakland Park, FL: BIOflex Medical Magnets Inc; 1998. The application of static altering magnetic field in medicine. Institute for Biophysics and Ray-Research medical study: Vienna, Austria [manufacturer's handout] [Google Scholar]
- 6.Hong CZ, Harmon D. Static magnetic field influence on rat tail nerve function. Arch Phys Med Rehabil. 1986;67:746–749. doi: 10.1016/0003-9993(86)90008-0. [DOI] [PubMed] [Google Scholar]
- 7.Hong CZ. Static magnetic field influence on human nerve function. Arch Phys Med Rehabil. 1987;68:162–164. [PubMed] [Google Scholar]
- 8.Cavopol AV, Wamil AW, Holcomb RR, McLean MJ. Measurement and analysis of static magnetic fields that block action potentials in cultured neurons. Bioelectromagnetics. 1995;16:197–206. doi: 10.1002/bem.2250160308. [DOI] [PubMed] [Google Scholar]
- 9.McLean MJ, Holcomb RR, Wamil AW, Pickett JD, Cavopol AV. Blockade of sensory neuron action potentials by a static magnetic field in the 10 mT range. Bioelectromagnetics. 1995;16:20–32. doi: 10.1002/bem.2250160108. [DOI] [PubMed] [Google Scholar]
- 10.Hong CZ, Lin JC, Bender LF, Schaffer JN, Meltzer RJ, Causin P. Magnetic necklace: its therapeutic effectiveness on neck and shoulder pain. Arch Phys Med Rehabil. 1982;63:462–466. [PubMed] [Google Scholar]
- 11.Leclaire R, Bourgouin J. Electromagnetic treatment of shoulder periarthritis: a randomized control trial of the efficiency and tolerance of magnetotherapy. Arch Phys Med Rehabil. 1991;72:284–287. [PubMed] [Google Scholar]
- 12.Vallbona C, Hazlewood CF, Jurida G. Response of pain to static magnetic fields in postpolio patients: a double blind study. Arch Phys Med Rehabil. 1997;78:1200–1203. doi: 10.1016/s0003-9993(97)90332-4. [DOI] [PubMed] [Google Scholar]
- 13.Laser T, Martin J. Oakland Park, FL: BIOflex Medical Magnets Inc; 1998. Double blind study of the therapeutic effectiveness of permanently magnetizing foils on secondary myotendofasciaopathies at different selected occasions. Klinic Bavaria Medical Study [manufacturer's handout] [Google Scholar]
- 14.Seaman L. Oakland Park, FL: BIOflex Medical Magnets Inc; 1998. A double-blind study demonstrating therapeutic benefit in heel pain symptomatology. Barry University Medical Study. Miami, FL [manufacturer's handout] [Google Scholar]
- 15.Wahlstrom O. Stimulation of a fracture healing with electromagnetic fields of extremely low frequency (EMF of ELF) Clin Orthop. 1984;186:293–301. [PubMed] [Google Scholar]
- 16.Sharrard WJ. A double-blind trial of pulsed electromagnetic fields for delayed union of tibial fractures. J Bone Joint Surg Br. 1990;72:347–355. doi: 10.1302/0301-620X.72B3.2187877. [DOI] [PubMed] [Google Scholar]
- 17.Bruce GK, Howlett CR, Huckstep RL. Effect of static magnetic field on fracture healing in a rabbit radius: preliminary results. Clin Orthop. 1987;222:300–306. [PubMed] [Google Scholar]
- 18.Trock DH, Bollet J, Dyer RH, Fielding P, Miner WK, Markoll R. A double blind trial of the clinical effects of pulsed electromagnetic fields in osteoarthritis. J Rheumatol. 1993;20:456–460. [PubMed] [Google Scholar]
- 19.Trock DH, Bollet AJ, Markoll R. The effect of pulsed electromagnetic fields in the treatment of osteoarthritis of the knee and cervical spine: report of randomized, double blind, placebo controlled trials. J Rheumatol. 1994;21:1903–1911. [PubMed] [Google Scholar]
- 20.Zablotsky TJ. Oakland Park, FL: BIOflex Medical Magnets Inc; 1998. The application of permanent magnets in musculoskeletal injuries [manufacturer's handout] [Google Scholar]
- 21.Pratt GW, Mishra L. The effect of BIOflex magnetic pads on the flow of 5% aqueous saline solution. Paper presented at: Massachusetts Institute of Technology International Symposium on Biomagnetism; May 29, 1989; Newport, RI. [Google Scholar]
- 22.Wolfson R, Pasachoff JM. New York, NY: Harper Collins; 1990. Physics Extended with Modern Physics; p. 713. [Google Scholar]
- 23.Los Angeles, CA: Nikken Inc; 1999. Advanced Magnet Technology. [manufacturer's handout] [Google Scholar]
- 24.Columbus Instrument International Corporation. Iso-Thermex Electronic Thermometer Instruction Manual. Columbus, OH: Columbus Instrument International Corp; 1985. [Google Scholar]
- 25.Michael ED, Katch FI. Prediction of body density from skinfold and girth measurements of 17-year-old boys. J Appl Physiol. 1968;25:747–750. doi: 10.1152/jappl.1968.25.6.747. [DOI] [PubMed] [Google Scholar]
- 26.Merrick MA, Knight KL, Ingersoll CD, Potteiger JA. The effects of ice and compression wraps on intramuscular temperatures at various depths. J Athl Train. 1993;28:236–245. [PMC free article] [PubMed] [Google Scholar]
- 27.Cidex-Plus [ package insert] Arlington, TX: Johnson & Johnson Medical Inc; 1999. [Google Scholar]
- 28.Ardizone V. Los Angeles, CA: Nikken Inc; 1992. Handouts for magnetic therapeutic conferences [manufacturer's handout] [Google Scholar]
- 29.Prentice W. Therapeutic Modalities in Sports Medicine. 3rd ed. St Louis, MO: Mosby; 1994. pp. 175–214. [Google Scholar]
- 30.Baker R, Bell G. The effect of therapeutic modalities on blood flow in the human calf. J Orthop Sports Phys Ther. 1991;13:23–27. doi: 10.2519/jospt.1991.13.1.23. [DOI] [PubMed] [Google Scholar]
- 31.Starkey C. Therapeutic Modalities for Athletic Trainers. Philadelphia, PA: FA Davis; 1993. pp. 60–68. [Google Scholar]
- 32.Lehmann JF, Warren CG, Scham SM. Therapeutic heat and cold. Clin Orthop. 1974;99:207–245. doi: 10.1097/00003086-197403000-00028. [DOI] [PubMed] [Google Scholar]
- 33.Baermann HM. The influence of multipolar static magnet field on the electrolytic system of a living organism with special reference to circulation and radial pole patterns. Paper presented at: International Symposium on Biomagnetology, Magnetotherapy and Postural Activity; May 29, 1989; Newport, RI. [Google Scholar]
- 34.White J. Alternative sports medicine. Physician Sportsmed. 1998;26(6):92–102. doi: 10.3810/psm.1998.06.1066. [DOI] [PubMed] [Google Scholar]
- 35.McEnroe C, Magnet PI. Men's Health. 1998;13:86–87. [Google Scholar]
- 36.Anderson G. The case for magnetic field therapy. Coach Athl Dir. 1997;66:34. [Google Scholar]
- 37.Tenforde TS. Thermoregulation in rodents exposed to high-intensity stationary magnetic fields. Bioelectromagnetics. 1986;7:341–346. doi: 10.1002/bem.2250070310. [DOI] [PubMed] [Google Scholar]