Abstract
Objective:
To investigate the relationship between the amount of overlying adipose and intramuscular temperature change during and after a 20-minute crushed-ice pack treatment.
Design and Setting:
Subjects were divided into 3 equal groups according to calf skinfold thickness: 8 mm or less, 10 to 18 mm, and 20 mm or greater. Intramuscular temperature was monitored at 1 cm and 3 cm below the subcutaneous fat in the left medial calf during and after a 1.8-kg crushed-ice pack treatment.
Subjects:
Thirty uninjured college students volunteered to be subjects.
Measurements:
Intramuscular temperature was recorded every 10 seconds over a 20-minute treatment and for 30 minutes posttreatment.
Results:
Intramuscular temperature decreases between adipose groups at the end of treatment at both 1 cm and 3 cm below the subcutaneous fat were significantly different. At 1 cm within the muscle, the temperature decreases were 14.43°C, 9.06°C, and 5.00°C for 8-mm or less, 10- to 18-mm, and 20-mm or greater skinfolds, respectively. At 3 cm, temperatures were 6.22°C, 3.86°C, and 2.42°C, respectively. By 30 minutes posttreatment at 1 cm, the 8-mm or less and 10- to 18-mm groups rewarmed 5.39°C and 2.22°C, respectively, but the 20-mm or greater group was 0.49°C colder than at the conclusion of the treatment. At 3 cm, temperatures in all 3 groups were colder at 30 minutes posttreatment than they were at the end of the treatment, 1.63°C, 1.83°C, and 2.10°C for 8-mm or less, 10- to 18-mm, and 20-mm or greater skinfolds, respectively.
Conclusions:
The amount of adipose over the therapy site is a significant factor in the extent of intramuscular temperature change that occurs during and after cryotherapy. Adipose should, therefore, be taken into account in determining appropriate treatment protocols.
Keywords: ice, fat, cooling, rewarming
In ancient times, Hippocrates advocated the therapeutic use of ice and snow.1,2 An athletic trainer, Don Bennett, in an article appearing in the 1961 winter issue of Athletic Training, has been credited with publishing “one of the first, if not the very first, mention of cold therapy for athletic injuries in the American literature.”3 Not long after, Grant4 and Hayden5 published their classic articles on cryokinetics. Today, cryotherapy, the therapeutic use of cold, is the focal point for the immediate management of musculoskeletal injury, and cryokinetics, alternating ice and exercise, is frequently used during the rehabilitation of these injuries.1,2,6–9
Cryotherapy has been shown to minimize the inflammatory response of acute musculoskeletal injury while maximizing functional recovery.7 When applying ice, a temperature gradient is obvious, with the skin cooling immediately, subcutaneous tissue next, and a delayed response in the muscle.1,7,8,10–12 Myrer et al13 developed a list of primary and secondary factors that account for the amount of intramuscular temperature change brought about by the use of superficial heat or cold modalities. The primary factors are the temperature gradient between the agent applied and the body surface to which it is applied,1,2,10,12,14–17 the region and surface area over which the agent is applied,2,10,12,15,18 the duration of the treatment,1,2,7,10,14–17 and the depth at which the tissue temperature is measured.1,2,15,17,19 The secondary factors are the rate of blood flow to the tissue and the local metabolic rate,7,12,20–23 individual variability,10,24,25 the sympathetic vasomotor integrity,7,12 and the amount of integument surrounding the muscle.2,7,8,10,14,17,24–26
Many researchers have written of the relative insulating value of fat,* but their findings with regard to cryotherapy have not been in agreement. Cheek and abdominal skinfold measurements, as well as somatotype assessment, did not correlate well with intramuscular temperature change in the gastrocnemius.12 A recent study27 examined the relationship between both body fat percentage and calf subcutaneous thickness with intramuscular temperature change due to ice massage and ice bag therapy. The authors concluded, “…the results of this study do not support the hypothesized insulating effects of subcutaneous fat during cryotherapy treatment in a sample of intercollegiate athletes.”27 Other research on the effect of overall percentage of body fat24 and site-specific adipose14 has demonstrated a significant inverse relationship between the amount of subcutaneous fat and intramuscular temperature change. Merrick et al26 stated, “The thickness of the adipose tissue is a major point in determining the rate of temperature decrease, as well as the absolute temperature decrease.” In spite of the general acceptance of this statement, we were unable to find any controlled research that specifically examined the relationship between the amount of subcutaneous adipose and intramuscular temperature change via cryotherapy over a relatively wide range of skinfold measurements. To fill this void, the purpose of our study was to investigate the relationship between the amount of overlying adipose and intramuscular temperature change during a 20-minute application of a crushed-ice pack treatment and for 30 minutes posttreatment.
METHODS
Subjects
Thirty healthy college students (12 women and 18 men; age, 24.3 ± 5.2 years; height, 175.1 ± 8.3 cm; weight, 70.8 ± 10.7 kg; calf skinfold thickness, 15.5 ± 8.7 mm, range, 3.8 to 35.3 mm) volunteered and signed the University Institutional Review Board–approved consent form to become subjects. The Board also approved the study.
Procedures
Any subject with a history of peripheral vascular disease or allergy to cephalexin hydrochloride was excluded from the study. We measured the skinfold thickness of the posterior left lower leg, at the visually determined greatest girth, using a Lange Skinfold Caliper (Cambridge Scientific Industries, Ltd, Cambridge, MD) with the subject standing and the weight borne on the right leg. Subjects were divided into 3 equal groups according to the skinfold measurement: group 1, 8 mm or less (6.5 ± 1.4 mm); group 2, 10 to 18 mm (14.1 ± 2.6 mm); group 3, 20 mm or greater (25.7 ± 5.4 mm). The skinfold measurement was divided by 2 to determine the depth of subcutaneous fat over each subject's gastrocnemius.13
To minimize the risk of infection from the insertion of the hypodermic needle microprobes into the intramuscular tissue, each subject took one 500-mg dose of cephalexin hydrochloride immediately before the experiment and 3 similar doses at 6-hour intervals at the conclusion of the experiment.13 Subjects assumed a prone position on a standard examining table. We cleansed a 4 × 4–cm area of skin over the midportion of the muscle belly of the left calf, first with a 10% povidone-iodine swab and then with a 70% isopropyl alcohol prep pad. Before and after each use, the 26-gauge hypodermic needle microprobes (Physitemp MT-26/2 and MT-26/4, Physitemp Instruments, Inc, Clifton, NJ) were washed with soap and water. We then performed high-level disinfection of the hypodermic needle microprobes by placing them in Cidex (Johnson & Johnson, New Brunswick, NJ) for at least 40 minutes and washed the Cidex from the probes with sterile water.
Using sterile technique, an advanced-practice registered nurse (G.J.M.) placed the 2 probes at 1 cm (MT-26/2) and 3 cm (MT-26/4), respectively, beneath the subcutaneous fat in the left medial calf. Insertion depths were controlled by marking on the medial side of the calf the appropriate vertical distance [0.5(skinfold measurement) + 1 or 3 cm, respectively] from the posterior surface of the lower leg using a caliper and inserting the microprobes parallel to the frontal plane.13 The probes were secured at the insertion sites with a piece of Transpore clear tape (3M Health Care, St Paul, MN) measuring 2.54 cm × 3.75 cm. The probes were then connected to an electronic thermocouple (Columbus Instruments, Iso-Thermex 16-channel, Columbus, OH) and, after 3 minutes, the baseline intramuscular temperature was recorded. After the baseline temperature was recorded, we placed a 1.8-kg crushed-ice pack (approximately 25 cm × 30 cm × 5 cm) directly over the triceps surae muscle group of each subject for 20 minutes. We recorded intramuscular temperature, to the nearest 0.01°C, every 10 seconds over the entire 20-minute treatment and for 30 minutes posttreatment.
After the probes were removed, the limb was dried and swabbed with a 70% isopropyl alcohol prep pad. To ensure that ambient room temperature did not affect muscle temperature, room temperature was monitored during the experiment using a thermocouple (Model TX-31, Columbus Instruments) interfaced to the Iso-Thermex.
Data Analysis
Our independent variables were adipose group, depth of measurement, and time. Our dependent variable was temperature. Although temperature measurements were available every 10 seconds, the measurements of primary interest were taken at baseline (T0), end of ice treatment (T20), and 30 minutes after ice treatment (T50). As a preliminary test to identify the existence of overall differences between adipose groups, we performed a multivariate analysis of variance (MANOVA) on the 3 time points of primary interest gathered for each subject. We calculated temperature change from baseline (T0) to the end of the treatment (T20) and from T20 to the end of the posttreatment period (T50). Analysis of variance (ANOVA) was used to determine if differences in mean temperature changes between adipose groups existed at each depth during treatment (T0–T20) and posttreatment (T20–T50). When differences were found, we used the Duncan multiple-range tests post hoc to determine where these differences occurred. An α level of P < .05 was used for all statistical analyses. We also calculated rates of temperature change for each adipose group at both depths over the treatment and posttreatment. Differences between adipose groups were examined by the use of t tests. To account for the multiple t tests, a Bonferroni correction factor was employed. We also determined each adipose group's lowest temperature and the time when it occurred.
RESULTS
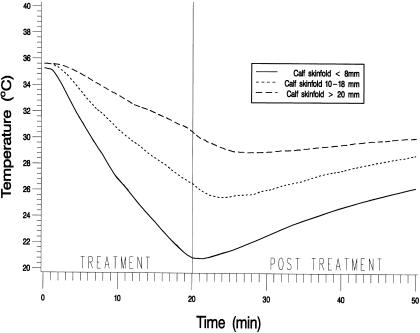
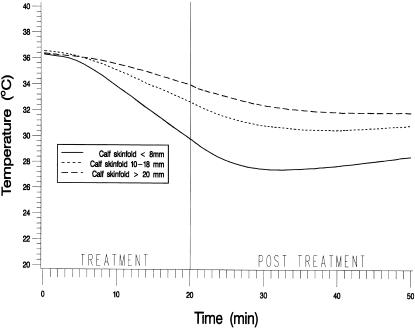
The MANOVA revealed significant differences in temperature change over time among adipose groups (F2,27 = 5.63, P < .0091) and depths (F1,27 = 33.84, P < .0001) and a nonsignificant group-by-depth interaction (F2,27 = 0.54, P = .5906). Figure 1 shows mean intramuscular temperatures throughout the treatment and the posttreatment period for the 3 adipose groups at 1 cm below the subcutaneous fat. Figure 2 shows the same information at 3 cm below the subcutaneous fat. Table 1 presents the means and standard deviations of the intramuscular temperature changes for the 3 adipose groups from T0 to T20, from T20 to T50, and from T0 to T50, for both depths. Table 2 presents the means and standard deviations of maximum temperature decrease and time to maximum decrease from the beginning of treatment by adipose group and depth. Table 3 presents the rate of temperature change per minute for each adipose group during the treatment and the posttreatment period.
Figure 1.
Mean intramuscular temperatures recorded at 10-second intervals throughout the treatment and the posttreatment period for the 3 adipose groups at 1 cm below the subcutaneous fat.
Figure 2.
Mean intramuscular temperatures recorded at 10-second intervals throughout the treatment and the posttreatment period for the 3 adipose groups at 3 cm below the subcutaneous fat.
Table 1.
Temperature Changes by Adipose Group, Depth of Measurement and Time*
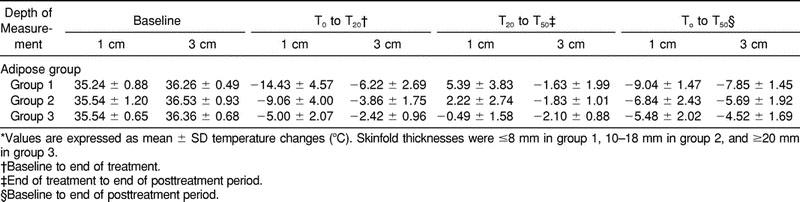
Table 2.
Maximum Temperature Decrease and Time to Maximum Temperature Decrease From Beginning of Treatment by Adipose Group and Depth of Measurement*
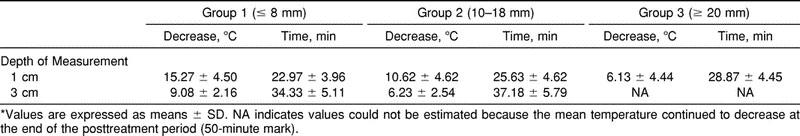
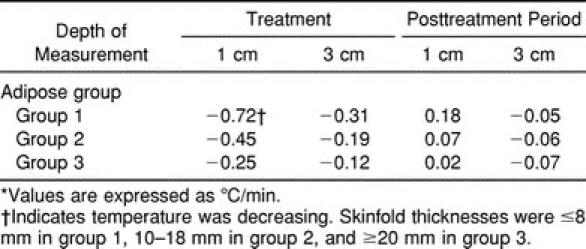
Table 3.
Rate of Temperature Change by Adipose Group and Depth of Measurement During Treatment and the Posttreatment Period*
Treatment
The ANOVAs showed significant intramuscular temperature differences among adipose groups at both the 1-cm (F2,27 = 16.35, P < .0001) and 3-cm (F2,27 = 9.78, P < .0006) depths during the treatment. The Duncan multiple-range tests revealed significant differences among all 3 adipose groups in temperatures at the 1-cm depth. At the 3-cm depth, however, temperatures in adipose group 1 were significantly different from temperatures in groups 2 and 3, but temperatures in groups 2 and 3 were not significantly different from each other.
A significant difference in the rate of temperature decrease among adipose groups at the 1-cm depth was noted (Table 3). With the Bonferroni correction, the rate of decrease for group 1 was significantly greater than that for group 3. The difference between the rate of decrease between groups 1 and 2 approached significance. At the 3-cm depth, the rate of decrease for group 1 was significantly greater than the rates of decrease for both groups 2 and 3. The rates of decrease in groups 2 and 3 were not significantly different from each other.
Posttreatment Period
Significant intramuscular temperature differences were seen among adipose groups at the 1-cm depth (F2,27 = 10.52, P < .0004) but not at the 3-cm depth (F2,27 = 0.29, P = .7538). For the 1-cm depth, the Duncan multiple-range tests revealed that the temperature change during the posttreatment period was not significantly different between groups 1 and 2, but changes were significantly different from those in group 3. The rate of intramuscular temperature change per minute during the posttreatment period at 1 cm below the subcutaneous fat revealed only that the rate of change in adipose group 1 was significantly different from that in group 3.
The rate of temperature change at 3 cm below the subcutaneous fat was not significantly different among any of the adipose groups. The room temperature of the laboratory varied little during the time we collected data (24.17° ± 0.32°C).
DISCUSSION
Clinically, cryotherapy is applied topically, but the muscles or ligaments situated below the integument are almost always the target tissues. Zemke et al,27 stated in their introduction that “subcutaneous fat at a treatment site may influence not only the lowest temperature achieved during and after the application of the cryotherapy mode, it may also affect the rate of change in intramuscular temperature and may play a role in the duration of intramuscular temperature depression.” They failed to prove these assertions and suggested that the narrow range of body fat percentage and their small sample size may have limited the generalizability of their results. Our results clearly indicate that, when examined over a relatively wide range of skinfold thicknesses (3.8 to 35.3 mm), subcutaneous fat is a significant factor in the magnitude and rate of intramuscular cooling during cryotherapy treatment and rewarming after treatment. Our results agree with previous research indicating that a significant inverse relationship exists between the amount of subcutaneous fat and intramuscular temperature change.14,24,26 There was a significant inverse relationship between skinfold thickness, or overlying adipose, and the magnitudes and rates of intramuscular temperature change at the 1-cm depth during both the treatment and the posttreatment period. At 1 cm below the subcutaneous fat, group 1 (8-mm or less skinfold) experienced an intramuscular temperature decrease of 14.43°C at a rate of 0.72°C /min. Group 3 (20-mm or greater skinfold) had decreases in rates and magnitudes of intramuscular temperature approximately one third of those of group 1 (decrease, 5.00°C; rate, 0.25°C/min). Group 2 (10- to 18-mm skinfold) had values very close to the means of groups 1 and 3 (decrease, 9.06°C; rate, 0.45°C/min). For all groups, the intramuscular temperature continued to decrease after the cryotherapy treatment had ceased. This finding is also in agreement with most of the previous research.1,7,11,24,26–28 The more overlying adipose, the greater the amount of time that was required in the posttreatment period before maximum cooling was obtained (Table 2). In fact, we were unable to establish the minimum temperature for group 3 at 3 cm below the subcutaneous fat because the muscle continued to get colder over the entire 30-minute posttreatment period. At the 1-cm depth, by the end of the 30-minute posttreatment period, temperatures in groups 1 and 2 rose above what they were at the end of the treatment, but temperatures in group 3 remained below values at the end of treatment.
The depth of the target tissue is also an important factor in cryotherapy.1,2,15,17,19 The deeper the target tissue, the longer it takes to cool and rewarm the tissue. Across adipose groups, the intramuscular tissue at 3 cm cooled to approximately 45% of the temperature of the muscle at 1 cm below the subcutaneous fat by the end of the 20-minute treatment. With increasing muscle depth, the overlying adipose appears to be less of a factor: only in group 1 were the magnitude and rate of temperature decrease significantly different from those in groups 2 and 3 during the treatment. At our deeper depth (3 cm into the muscle), after the medium amount of overlying adipose was reached (group 2), further amounts of adipose apparently had little effect on the rate or magnitude of temperature change. No difference in rewarming was found among adipose groups at the 3-cm depth.
Although the exact therapeutic time range needed to ensure the benefits of cryotherapy has not as yet been determined, the secondary injury models suggest that the sooner the target tissue temperature is lowered, the less secondary injury will occur.29,30 Our results indicate that the amount of adipose over the target tissue significantly affects both the rate and magnitude of cooling that take place in the underlying target tissue and, therefore, is an important factor in the efficacy of cryotherapy treatment.
CONCLUSIONS
A significant inverse relationship exists between overlying adipose and intramuscular temperature change during and after cryotherapy treatment when examined over a relatively wide range of skinfold thicknesses. It appears that overlying adipose becomes less of a factor the deeper into the target tissue one goes. Therefore, the depth of the target tissue and the amount of overlying adipose should be accounted for in determining treatment time when applying cryotherapy.
Footnotes
REFERENCES
- 1.Meeusen R, Lievens P. The use of cryotherapy in sports injuries. Sports Med. 1986;3:398–414. doi: 10.2165/00007256-198603060-00002. [DOI] [PubMed] [Google Scholar]
- 2.Swenson C, Sward L, Karlsson J. Cryotherapy in sports medicine. Scand J Med Sci Sports. 1996;6:193–200. doi: 10.1111/j.1600-0838.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 3.Barnes L. Cryotherapy—putting injury on ice. Physician Sportsmed. 1979;7(6):130–136. doi: 10.1080/00913847.1979.11710883. [DOI] [PubMed] [Google Scholar]
- 4.Grant AE. Massage with ice (cryokinetics) in treatment of painful conditions of the musculoskeletal system. Arch Phys Med Rehabil. 1964;45:233–238. [PubMed] [Google Scholar]
- 5.Hayden CA. Cryokinetics in an early treatment program. J Am Phys Ther Assoc. 1964;44:990–993. doi: 10.1093/ptj/44.11.990. [DOI] [PubMed] [Google Scholar]
- 6.Kowal MA. Review of physiological effects of cryotherapy. J Orthop Sports Phys Ther. 1983;5:66–73. doi: 10.2519/jospt.1983.5.2.66. [DOI] [PubMed] [Google Scholar]
- 7.Halvorson GA. Therapeutic heat and cold for athletic injuries. Physician Sportsmed. 1990;18(5):87–94. doi: 10.1080/00913847.1990.11710045. [DOI] [PubMed] [Google Scholar]
- 8.Kaul MP, Herring SA. Superficial heat and cold. Physician Sportsmed. 1994;22(12):65–74. doi: 10.1080/00913847.1994.11947720. [DOI] [PubMed] [Google Scholar]
- 9.McMaster WC, Liddle S, Waugh TR. Laboratory evaluation of various cold therapy modalities. Am J Sports Med. 1978;6:291–294. doi: 10.1177/036354657800600513. [DOI] [PubMed] [Google Scholar]
- 10.Bierman W, Friediander M. The penetrative effect of cold. Arch Phys Ther. 1940;21:585–592. [Google Scholar]
- 11.Hartviksen K. Ice therapy in spasticity. Acta Neurol Scand. 1962;38(suppl 3):79–84. [PubMed] [Google Scholar]
- 12.Wolf SL, Basmajian JV. Intramuscular temperature changes deep to localized cutaneous cold stimulation. Phys Ther. 1979;53:1284–1288. doi: 10.1093/ptj/53.12.1284. [DOI] [PubMed] [Google Scholar]
- 13.Myrer JW, Draper DO, Durrant E. Contrast therapy and intramuscular temperature in the human leg. J Athl Train. 1994;29:318–322. [PMC free article] [PubMed] [Google Scholar]
- 14.Lowdon BJ, Moore RJ. Determinants and nature of intramuscular temperature changes during cold therapy. Am J Phys Med. 1975;54:223–233. [PubMed] [Google Scholar]
- 15.Lehmann JF, Warren CG, Scham SM. Therapeutic heat and cold. Clin Orthop. 1974;99:207–245. doi: 10.1097/00003086-197403000-00028. [DOI] [PubMed] [Google Scholar]
- 16.Barcroft H, Edholm OG. The effect of temperature on blood flow and deep temperature in the human forearm. J Physiol. 1943;102:5–20. doi: 10.1113/jphysiol.1943.sp004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myrer JW, Measom G, Durrant E, Fellingham GW. Cold- and hot-pack contrast therapy: subcutaneous and intramuscular temperature change. J Athl Train. 1997;32:238–241. [PMC free article] [PubMed] [Google Scholar]
- 18.Lehmann JF. Therapeutic Heat and Cold. 4th ed. Baltimore, MD: Williams & Wilkins; 1990. pp. 590–632. [Google Scholar]
- 19.Waylonis GW. The physiologic effects of ice massage. Arch Phys Med Rehabil. 1967;48:37–42. [PubMed] [Google Scholar]
- 20.Ho SSW, Coel MN, Kagawa R, Richardson AB. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537–540. doi: 10.1177/036354659402200417. [DOI] [PubMed] [Google Scholar]
- 21.Ho SSW, Illgen RL, Meyer RW, Torok PJ, Cooper MD, Reider B. Comparison of various icing times in decreasing bone metabolism and blood flow in the knee. Am J Sports Med. 1995;23:74–76. doi: 10.1177/036354659502300112. [DOI] [PubMed] [Google Scholar]
- 22.Thorsson O, Lilja B, Ahlgren L, Hemdal B, Westlin N. The effect of local cold application on intramuscular blood flow at rest and after running. Med Sci Sports Exerc. 1985;17:710–713. doi: 10.1249/00005768-198512000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Morvan D, Leroy-Willig A, Malgouyres A, Cuenod CA, Jehenson P, Syrota A. Simultaneous temperature and regional blood volume measurements in human muscle using an MRI fast diffusion technique. Magn Reson Med. 1993;29:371–377. doi: 10.1002/mrm.1910290313. [DOI] [PubMed] [Google Scholar]
- 24.Johnson DJ, Moore S, Moore J, Oliver RA. Effect of cold submersion on intramuscular temperature of the gastrocnemius muscle. Phys Ther. 1979;59:1238–1242. doi: 10.1093/ptj/59.10.1238. [DOI] [PubMed] [Google Scholar]
- 25.Bing HI, Carlsten A, Christiansen SV. The effect on muscular temperature produced by cooling normal and ultraviolet radiated skin. Acta Med Scand. 1945;121:577–591. doi: 10.1111/j.0954-6820.1945.tb06900.x. [DOI] [PubMed] [Google Scholar]
- 26.Merrick MA, Knight KL, Ingersoll CD, Potteiger JA. The effects of ice and compression wraps on intramuscular temperatures at various depths. J Athl Train. 1993;28:236–245. [PMC free article] [PubMed] [Google Scholar]
- 27.Zemke JE, Andersen JC, Guion WK, McMillan J, Joyner AB. Intramuscular temperature responses in the human leg to two forms of cryotherapy: ice massage and ice bag. J Orthop Sports Phys Ther. 1998;27:301–307. doi: 10.2519/jospt.1998.27.4.301. [DOI] [PubMed] [Google Scholar]
- 28.Myrer JW, Measom G, Fellingham GW. Temperature changes in the human leg during and after two methods of cryotherapy. J Athl Train. 1998;33:25–29. [PMC free article] [PubMed] [Google Scholar]
- 29.Knight KL. Cryotherapy in Sports Injury Management. Champaign, IL: Human Kinetics; 1995. pp. 3–98. [Google Scholar]
- 30.Merrick MA, Rankin JM, Andres FA, Hinman CL. A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Med Sci Sports Exerc. 1999;31:1516–1521. doi: 10.1097/00005768-199911000-00004. [DOI] [PubMed] [Google Scholar]