Abstract
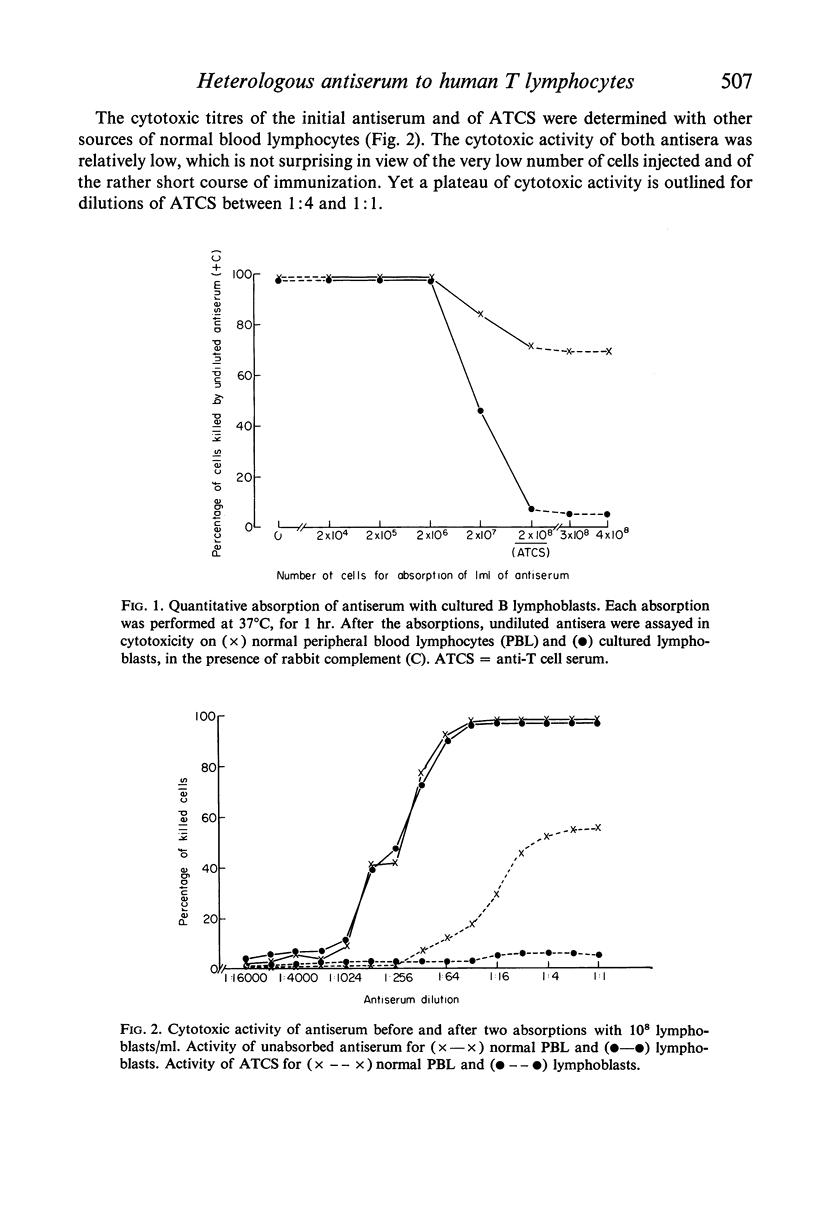
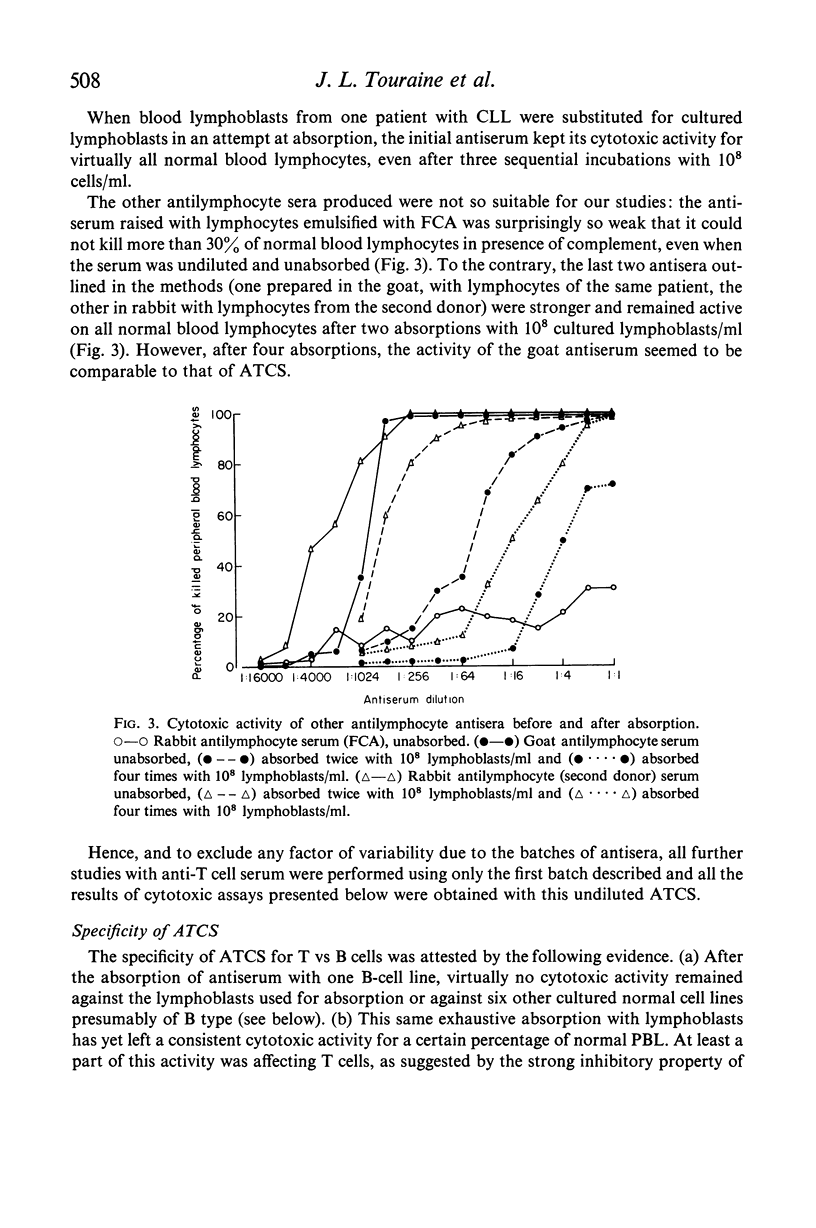
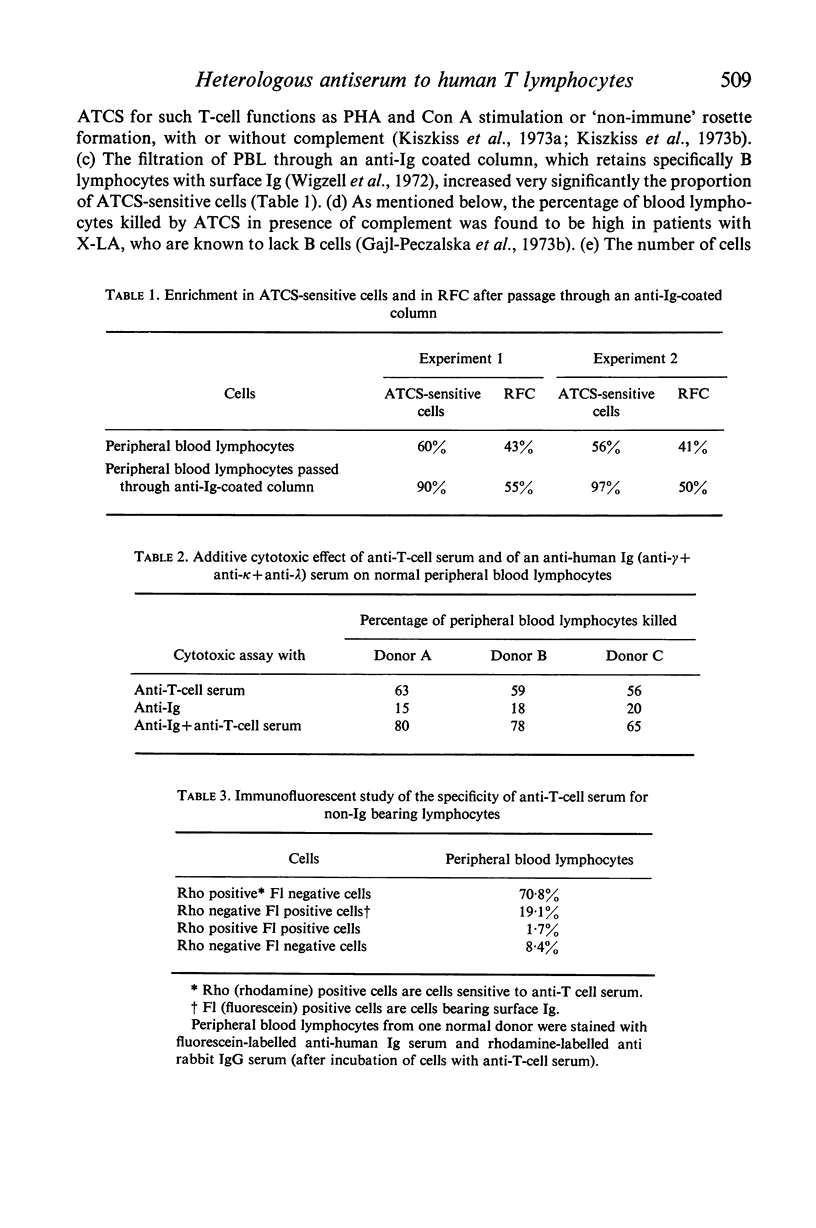
Peripheral blood lymphocytes (PBL) from two patients with Bruton-type agammaglobulinaemia were used for the preparation of heterologous anti-human T cell-sera which were absorbed with cultured B lymphoblast cells, peripheral blood lymphocytes from a patient with chronic lymphatic leukaemia and `adherent' cells. Using multiple criteria, one antiserum (ATCS) was shown to be specific for T lymphocytes. This antiserum asserts the existence of human-specific T-lymphocyte antigen(s) (HTLA) and provides another method for identifying human T cells. In the presence of rabbit complement, ATCS was cytotoxic for 65·5% (range 49–78) of normal PBL and 97% of thymocytes (the latter cells having also a higher surface density of HTLA than PBL). The study of PBL from a variety of patients showed that the percentage of ATCS-sensitive cells was high in Bruton-type agammaglobulinaemia, variable from patient to patient and from time to time in common variable hypogammaglobulinaemia and generally low in active lepromatous leprosy, in patients under antilymphocyte globulin therapy and in chronic lymphatic leukaemia. Cultured lymphoblasts from various B cell lines or from a Burkitt lymphoma cell line were resistant to ATCS.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablin R. J., Morris A. J. Thymus-specific antigens on human thymocytes and on thymic-derived lymphocytes. Transplantation. 1973 Apr;15(4):415–417. doi: 10.1097/00007890-197304000-00014. [DOI] [PubMed] [Google Scholar]
- Aisenberg A. C., Bloch K. J., Long J. C., Colvin R. B. Reaction of normal human lymphocytes and chronic lymphocytic leukemia cells with an antithymocyte antiserum. Blood. 1973 Mar;41(3):417–423. [PubMed] [Google Scholar]
- Aiuti F., Wigzell H. Function and distribution pattern of human T lymphocytes. I. Production of anti-T lymphocyte specific sera as estimated by cytotoxicity and elimination of function of lymphocytes. Clin Exp Immunol. 1973 Feb;13(2):171–181. [PMC free article] [PubMed] [Google Scholar]
- Aiuti F., Wigzell H. Function and distribution pattern of human T lymphocytes. II. Presence of T lymphocytes in normal humans and in humans with various immunodeficiency disorders. Clin Exp Immunol. 1973 Feb;13(2):183–189. [PMC free article] [PubMed] [Google Scholar]
- Amos D. B., Bashir H., Boyle W., MacQueen M., Tiilikainen A. A simple micro cytotoxicity test. Transplantation. 1969 Mar;7(3):220–223. doi: 10.1097/00007890-196903000-00023. [DOI] [PubMed] [Google Scholar]
- Aoki T., Hämmerling U., De Harven E., Boyse E. A., Old L. J. Antigenic structure of cell surfaces. An immunoferritin study of the occurrence and topography of H-2' theta, and TL alloantigens on mouse cells. J Exp Med. 1969 Nov 1;130(5):979–1001. doi: 10.1084/jem.130.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J. F. Evaluation of T-cells and thymic serum factors in man using the rosette technique. Transplant Rev. 1973;16(0):196–217. doi: 10.1111/j.1600-065x.1973.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Bullock W. E., Jr, Fasal P. Studies of immune mechanisms in leprosy. 3. The role of cellular and humoral factors in impairment of the in vitro immune response. J Immunol. 1971 Apr;106(4):888–899. [PubMed] [Google Scholar]
- Choi Y. S., Biggar W. D., Good R. A. Biosynthesis and secretion of immunoglobulins by peripheral-blood lymphocytes in severe hypogammaglobulinaemia. Lancet. 1972 May 27;1(7761):1149–1152. doi: 10.1016/s0140-6736(72)91374-8. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., Bockman D. E. Agammaglobulinaemia with B lymphocytes. Specific defect of plasma-cell differentiation. Lancet. 1971 Oct 9;2(7728):791–794. doi: 10.1016/s0140-6736(71)92742-5. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Siegal F. P., Bentwich Z. H., Kunkel H. G. Lymphocyte binding of aggregated IgG and surface Ig staining in chronic lymphocytic leukaemia. Clin Exp Immunol. 1973 May;14(1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Dukor P., Bianco C., Nussenzweig V. Bone marrow origin of complement-receptor lymphocytes. Eur J Immunol. 1971 Dec;1(6):491–494. doi: 10.1002/eji.1830010617. [DOI] [PubMed] [Google Scholar]
- Elves M. W., Gough J., Israels M. C. The relationship between the lymphocyte and polymorph during macrophage formation in vitro. Exp Cell Res. 1966 Nov-Dec;44(2):624–627. doi: 10.1016/0014-4827(66)90468-x. [DOI] [PubMed] [Google Scholar]
- Gajl-Peczalska K. J., Lim S. D., Jacobson R. R., Good R. A. B lymphocytes in lepromatous leprosy. N Engl J Med. 1973 May 17;288(20):1033–1035. doi: 10.1056/NEJM197305172882001. [DOI] [PubMed] [Google Scholar]
- Gajl-Peczalska K. J., Park B. H., Biggar W. D., Good R. A. B and T lymphocytes in primary immunodeficiency disease in man. J Clin Invest. 1973 Apr;52(4):919–928. doi: 10.1172/JCI107257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzo J. J., Wood M. L., Monaco A. P. Use of minimal doses of lymphoid cells for production of heterologous antilymphocyte serum. Transplant Proc. 1971 Mar;3(1):779–783. [PubMed] [Google Scholar]
- Han S. H., Weiser R. S., Kau S. T. Prolonged survival of skin allografts in leprosy patients. Int J Lepr Other Mycobact Dis. 1971 Jan-Mar;39(1):1–6. [PubMed] [Google Scholar]
- Itakura K., Hutton J. J., Boyse E. A., Old L. J. Genetic linkage relationships of loci specifying differentiation alloantigens in the mouse. Transplantation. 1972 Mar;13(3):239–243. doi: 10.1097/00007890-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamelin J. P., Lisowska-Bernstein B., Matter A., Ryser J. E., Vassalli P. Mouse thymus-independent and thymus-derived lymphoid cells. I. Immunofluorescent and functional studies. J Exp Med. 1972 Nov 1;136(5):984–1007. doi: 10.1084/jem.136.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., Miller J. F. Cell to cell interaction in the immune response. IV. Site of action of antilymphocyte globulin. J Exp Med. 1968 Oct 1;128(4):855–874. doi: 10.1084/jem.128.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCredie K. B., Hersh E. M., Freireich E. J. Cells capable of colony formation in the peripheral blood of man. Science. 1971 Jan 22;171(3968):293–294. doi: 10.1126/science.171.3968.293. [DOI] [PubMed] [Google Scholar]
- Ohayon E., Ducos J., Goret P., Salmon H., Toma B. Cytotoxicity of A.L.S. and anti-HL-A antibodies in human leukaemia. Lancet. 1972 Jan 1;1(7740):39–40. doi: 10.1016/s0140-6736(72)90030-x. [DOI] [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piessens W. F., Schur P. H., Moloney W. C., Churchill W. H. Lymphocyte surface immunoglobulins. Distribution and frequency in lymphoproliferative diseases. N Engl J Med. 1973 Jan 25;288(4):176–180. doi: 10.1056/NEJM197301252880403. [DOI] [PubMed] [Google Scholar]
- Pincus S., Bianco C., Nussenzweig V. Increased proportion of complement-receptor lymphocytes in the peripheral blood of patients with chronic lymphocytic leukemia. Blood. 1972 Sep;40(3):303–310. [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Surface bound immunoglobulins as a cell marker in human lymphoproliferative diseases. Blood. 1972 Dec;40(6):777–794. [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Meyers O. L., David J. R. An in vitro assay for cellular hypersensitivity in man. J Immunol. 1970 Jan;104(1):95–102. [PubMed] [Google Scholar]
- Ross G. D., Rabellino E. M., Polley M. J., Grey H. M. Combined studies of complement receptor and surface immunoglobulin-bearing cells and sheep erythrocyte rosette-forming cells in normal and leukemic human lymphocytes. J Clin Invest. 1973 Feb;52(2):377–385. doi: 10.1172/JCI107194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabolović D., Sabolovic N., Dumont F. Identification of T and B cells in mouse and man. Lancet. 1972 Oct 28;2(7783):927–927. doi: 10.1016/s0140-6736(72)92567-6. [DOI] [PubMed] [Google Scholar]
- Shigeno N., Hämmerling U., Arpels C., Boyse E. A., Old L. J. Preparation of lymphocyte-specific antibody from anti-lymphocyte serum. Lancet. 1968 Aug 10;2(7563):320–323. doi: 10.1016/s0140-6736(68)90530-8. [DOI] [PubMed] [Google Scholar]
- Shortman K., Byrd W., Williams N., Brunner K. T., Cerottini J. C. The separation of different cell classes from lymphoid organs. The relationship between the adherence properties and the buoyant density of sub-populations of "B" and "T" lymphocytes. Aust J Exp Biol Med Sci. 1972 Jun;50(3):323–336. doi: 10.1038/icb.1972.26. [DOI] [PubMed] [Google Scholar]
- Shortman K., Williams N., Jackson H., Russell P., Byrt P., Diener E. The separation of different cell classes from lymphoid organs. IV. The separation of lymphocytes from phagocytes on glass bead columns, and its effect on subpopulations of lymphocytes and antibody-forming cells. J Cell Biol. 1971 Mar;48(3):566–579. doi: 10.1083/jcb.48.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. W., Terry W. D., Buell D. N., Sell K. W. An antigenic marker for human thymic lymphocytes. J Immunol. 1973 Mar;110(3):884–887. [PubMed] [Google Scholar]
- Stutman O., Good R. A. Heterogeneity of lymphocyte populations. Rev Eur Etud Clin Biol. 1972 Jan;17(1):11–14. [PubMed] [Google Scholar]
- Talwar G. P., Krishnan A. D., Mehra V. L., Blum E. A., Pearson J. M. Evaluation of cell mediated immune responses in untreated cases of leprosy. Clin Exp Immunol. 1972 Oct;12(2):195–203. [PMC free article] [PubMed] [Google Scholar]
- Thomas D. B. Antibodies to membrane antigen(s) common to thymocytes and a subpopulation of lymphocytes in infectious-mononucleosis sera. Lancet. 1972 Feb 19;1(7747):399–403. doi: 10.1016/s0140-6736(72)90854-9. [DOI] [PubMed] [Google Scholar]
- Touraine J. L., Revillard J. P., Brochier J., Manel A. M., Fries D., Traeger J. Le déficit de l'immunité cellulàire secondaire à l'insuffisance rénale. Lyon Med. 1970 Sep 27;224(30):185–200. [PubMed] [Google Scholar]
- Turk J. L., Waters M. F. Cell-mediated immunity in patients with leprosy. Lancet. 1969 Aug 2;2(7614):243–246. doi: 10.1016/s0140-6736(69)90009-9. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Waters M. F. Immunological basis for depression of cellular immunity and the delayed allergic response in patients with lepromatous leprosy. Lancet. 1968 Aug 24;2(7565):436–438. doi: 10.1016/s0140-6736(68)90472-8. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Sundqvist K. G., Yoshida T. O. Separation of cells according to surface antigens by the use of antibody-coated columns. Fractionation of cells carrying immunoglobulins and blood group antigen. Scand J Immunol. 1972;1(1):75–87. doi: 10.1111/j.1365-3083.1972.tb03737.x. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, DeBoard J. R., Mellbye O. J., Messner R. P., Lindström F. D. Studies of T- and B-lymphocytes in patients with connective tissue diseases. J Clin Invest. 1973 Feb;52(2):283–295. doi: 10.1172/JCI107184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Nossal G. J. Identification of human T and B lymphocytes in normal peripheral blood and in chronic lymphocytic leukaemia. Lancet. 1971 Oct 9;2(7728):788–791. doi: 10.1016/s0140-6736(71)92741-3. [DOI] [PubMed] [Google Scholar]
- Wood N., Bashir H., Greally J., Amos D. B., Yunis E. J. A simple method of freezing and storing live lymphocytes. Tissue Antigens. 1972;2(1):27–31. doi: 10.1111/j.1399-0039.1972.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Worth H. H., Cooper A. G., Brown M. C. Inhibition of human lymphocyte rosetting by anti-T sera. Nat New Biol. 1973 May 23;243(125):109–111. [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. The human rosette-forming cell as a marker of a population of thymus-derived cells. J Clin Invest. 1972 Oct;51(10):2537–2543. doi: 10.1172/JCI107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Chantler S., Fudenberg H. H. Isolation of normal T cells in chronic lymphatic leukaemia. Lancet. 1973 Jan 20;1(7795):126–129. doi: 10.1016/s0140-6736(73)90196-7. [DOI] [PubMed] [Google Scholar]


