Abstract
Two antimelanoma immunoconjugates containing a human single-chain Fv (scFv) targeting domain conjugated to the Fc effector domain of human IgG1 were synthesized as secreted two-chain molecules in Chinese hamster ovary and Drosophila S2 cells, and purified by affinity chromatography on protein A. The scFv targeting domains originally were isolated as melanoma-specific clones from a scFv fusion-phage library, derived from the antibody repertoire of a vaccinated melanoma patient. The purified immunoconjugates showed similar binding specificity as did the fusion-phage clones. Binding occurred to human melanoma cells but not to human melanocytes or to several other types of normal cells and tumor cells. A 250-kDa melanoma protein was immunoprecipitated by the immunoconjugates and analyzed by mass spectrometry, using two independent procedures. A screen of protein sequence databases showed an exact match of several peptide masses between the immunoprecipitated protein and the core protein of a chondroitin sulfate proteoglycan, which is expressed on the surface of most human melanoma cells. The Fc effector domain of the immunoconjugates binds natural killer (NK) cells and also the C1q protein that initiates the complement cascade; both NK cells and complement can activate powerful cytolytic responses against the targeted tumor cells. An in vitro cytolysis assay was used to test for an immunoconjugate-dependent specific cytolytic response against cultured human melanoma cells by NK cells and complement. The melanoma cells, but not the human fibroblast cells used as the control, were efficiently lysed by both NK cells and complement in the presence of the immunoconjugates. The in vitro results suggest that the immunoconjugates also could activate a specific cytolytic immune response against melanoma tumors in vivo.
One of the strategies for immunotherapy of cancer involves administering bifunctional reagents containing a tumor-targeting domain conjugated to an effector domain that can promote tumor regression. The tumor-targeting domain usually is derived from the combining site of an antitumor antibody (1). The effector domain can be a cytotoxic molecule such as Pseudomonas exotoxin, ricin, doxorubicin, or Diptheria toxin (2), or a molecule that can induce a cytolytic immune response, such as the Fc region of an Ig (3), the combining site of an anti-T cell antibody (4), or a bacterial superantigen (5). The clinical effectiveness of such bifunctional immunoconjugates is determined in large part by the tumor specificity of the targeting domain, which should be sufficiently stringent to avoid significant binding to normal cells, and by the immune rejection response induced in patients, which should be sufficiently weak to allow long-term administration of the immunoconjugate.
In the study reported here, we describe the properties of two human antimelanoma immunoconjugates that could have potential applications for melanoma immunotherapy. Each immunoconjugate contains a human single-chain Fv (scFv) molecule as the tumor-targeting domain, conjugated to the Fc region of a human IgG1 Ig as the effector domain, constituting virtually a human molecule that should be tolerated by the human immune system. The scFv molecules originally were isolated as melanoma-specific clones from fusion-phage libraries derived from the antibody repertoire of a melanoma patient who had been vaccinated with genetically modified autologous tumor cells (6). The melanoma-specific clones bind to human melanoma cell lines and frozen tissue sections, but do not bind to primary cultures of normal melanocytes, endothelial cells, and fibroblast cells, or to sections of 15 different normal human tissues or several tumors other than melanoma (7). The Fc region of human IgG1 binds the CD16 receptor on natural killer (NK) cells and also the C1q protein that initiates the complement cascade (4). Both NK cells and complement can trigger powerful cytolytic immune pathways, which should be directed against the melanoma cells targeted by the scFv domain of the immunoconjugate.
The immunoconjugates for this study were synthesized in mammalian and insect cells as homodimeric molecules, similar to a natural Camelid antibody that lacks a light chain and the C1 region of the heavy chain (8). The melanoma protein immunoprecipitated by the immunoconjugates was identified by mass spectrometric analyses as the core protein of a melanoma-associated chondroitin sulfate proteoglycan (MCSP) (9–11), which is expressed on the surface of most human melanoma cells (9–13). The results of in vitro cytotoxicity tests show that the immunoconjugates can specifically target human melanoma cells for lysis by NK cells and complement and therefore also might be effective against melanoma tumors in situ.
MATERIALS AND METHODS
Cell Cultures.
The permanent human melanoma lines A2058 (American Type Culture Collection) and TF2 (14) were grown in DMEM + 10% fetal calf serum. Primary cultures of human microvascular endothelial cells and fibroblast cells were extruded from newborn foreskin (15) and cultured in RPMI medium supplemented with 8% fetal bovine serum and 2% human peripartum serum; the endothelial cells were further supplemented with 33 mM 3-isobutyl-1-methylxanthene and 0.5 mM dibutyryl cAMP. Primary cultures of human melanocytes from newborn foreskin were prepared by the Skin Disease Research Center at the Yale University School of Medicine. The transformed human kidney cells 293-EBNA (Invitrogen) and the Chinese hamster ovary (CHO) cells were grown in RPMI + 10% fetal calf serum. Drosphila cells (Schneider S2) were grown at 25°C in Ex-cell 301 medium (JRH Biosciences, Lenexa, KS) + 10% fetal bovine serum. Resting NK cells were isolated from normal donors by leukophoresis and immunoselection (16) and were used within 18 hr after isolation; most of the cells (>97%) were CD3−, CD56+, and CD16+.
Preparation of the Immunoconjugates.
The procedures involved transfecting the expression vector pcDNA3.1 (Invitrogen) into CHO cells or the expression vector pMK33/pMtHy (gift from M. Koelle, Yale University) into Drosphila cells; each vector carried a cDNA encoding a secreted immunoconjugate (Fig. 1). The cDNAs for the IgG1 leader was synthesized by hybridizing two complementary oligonucleotides containing EcoRI and SacI ends, as follows: (a) AATTCATGGAGTTTGGGCTGAGCTGGCTTTTTCTTGTTGCTGCATTAAGAGGTGTCCAGTCCGAGCT; and (b) CGGACTGGACACCTGTTAATGCCAGCAACAAGAAAAGCCGCTCAGCCCAAACTCATG. The cDNA for the scFv targeting domains were synthesized from the corresponding fusion phase (7) by using one PCR prime containing a SacI site and another primer containing a BamHI site as follows: (a) GTCGAGCAGAGCTCCAGGTGCAGCTGGTGCAGTCTGGGGCTGAGGTGAGGTGAAGAAGCC; and (b) ACGTTCAGGGGATCCACCTAGGACGGTCAGCTTGGTCCC. The human Fc effector domain was synthesized from a cDNA library derived from human peripheral blood lymphocytes, using one PCR primer containing a BamHI site and another primer containing a SalI site as follows: (a) ACCTTGCAGGATCCGCAAGACCCAAATCTTGTGACAAAACTCAC, and (b) GATCACGTGTCGACTTATCATTTACCCGGAGACAGGGAGAGGCTCTTTCTG.
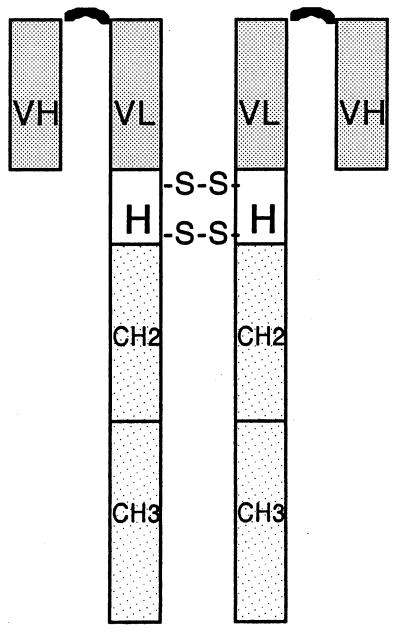
Figure 1.
Organization of the scFv targeting domain and Fc effector domain of an immunoconjugate molecule. VH and VL, heavy chain and light chain variable regions, respectively, derived from a scFv fusion-phage clone (7); a (G4S)3 polypeptide (curved line) links the VH to the VL region. H, hinge region of a human IgG1 Ig containing two disulfide bridges. CH2 and CH3, second and third constant regions of a human IgG1 Ig.
These three cDNAs encoding a secreted immunoconjugate were cloned sequentially into a vector for sequencing and then subcloned into the expression vectors pcDNA3.1 and pMK33/pMtHy for transfection into CHO or Drosophila S2 cells, respectively. The transfection procedure for CHO cells involved growing the cells in RPMI + 10% fetal calf serum and transfecting with 5 μg of an expression vector using Superfect (Qiagen, Chatsworth, CA). Stable transfectants were selected in RPMI + 10% fetal calf serum + 1 mg/ml of G418. For protein expression, transfected CHO cells first were adapted to growth in CHO serum-free medium (Sigma), and then were grown for 3 days as a suspension culture (2 × 105 cells/ml) in the serum-free medium. The transfection procedure for Drosophila S2 cells involved growing the cells in Ex-cell medium + 10% fetal bovine serum and transfecting with 10 μg of an expression vector using Lipofectin (GIBCO/BRL). Stable transfectants were selected in Ex-cell medium + 10% fetal bovine serum + 300 μg/ml of hygromycin, and adapted for growth as a suspension culture in serum-free Ex-cell medium. Expression of the encoded immunoconjugate was induced in the suspension culture by addition of 500 μM copper sulfate.
The immunoconjugates secreted by transfected CHO or Drosophila S2 cells were purified from the culture medium by affinity chromatography on a protein A matrix (Pierce).
Binding Specificity of the Immunoconjugates Measured by Fluorescence-Activated Cell Sorting (FACS).
Melanoma cells and control cells were harvested in nonenzymatic dissociation medium (Sigma), washed with PBS/BSA + 0.1% sodium azide, and incubated in PBS/BSA with added immunoconjugate (1 μg/ml) or in PBS/BSA without an immunoconjugate as a control. The cells were washed with PBS/BSA, incubated 30 min at 4°C with fluoroscein-labeled anti-human Fc γ-chain (Vector), and analyzed on a Becton-Dickenson FACsort instrument.
Immunoprecipitation of a Melanoma Cell Extract with an Immunoconjugate.
A sample of about 1 × 107 cells from the human melanoma line A2058 was suspended in a solution containing 10 μg/ml of immunoconjugate, 1% BSA, and 0.05% sodium azide in PBS and was incubated for 30 min on ice. The cells were washed twice with PBS and lysed in a solution containing 1% Nonidet P-40, 1 μg/ml of immunoconjugate, and 0.2 mM phenylmethylsulfonyl fluoride in PBS for 20 min on ice. The lysate was spun at 13,000 rpm in microfuge for 5 min, and the supernatant was recovered and incubated with protein-G beads overnight on a rotator. The beads were collected, washed twice with a solution containing 1% Nonidet P-40 in PBS and once with PBS, and then collected, boiled in PAGE loading buffer, and analyzed by PAGE.
Matrix-Assisted Laser Desorption Ionization-Mass Spectrometry (MALDI-MS) and Liquid Chromatography Tandem MS (LC/MS/MS) Protein Identification.
A protein band stained with Coomassie blue was excised from the gel and digested with trypsin as described at http://info.med.yale.edu/wmkeck/geldig3.htm. A sample of the tryptic digest was analyzed by MALDI-MS (17, 18) on a Micromass TofSpec SE. To attain the high level of accuracy needed for peptide mass searching, 100 fmol of bradykinin, which has a protonated monoisotpic mass of 1060.57, and andrenocorticotropin clip, which has a protonated monoisotopic mass of 2465.2, were used as internal calibrants. The resulting monoisotopic masses of the tryptic peptides were searched against the OWL database with the profound program using a mass tolerance of 0.2 Da, and against the European Molecular Biology Laboratory nonredundant database with the peptidesearch program using a 0.015% mass tolerance. Other important criteria used in the search were a mass range that extended from 140 to 560 kDa, a maximum of one missed cleavage, and no limitation with regard to taxonomy. All of the protein chemistry and mass spectronomy studies were carried out in the W.M. Keck Foundation and Howard Hughes Medical Institute Biopolymer Laboratory at Yale University. Further information can be found at http://info.med.yale.edu/wmkeck/.
A sample of the trypsin-digested protein band used for the matrix-assisted laser desorption ionization-MS analysis also was analyzed by liquid chromatography/MS/MS on a LCQ ion trap mass spectrometer (19). A sequest search of the MS/MS data was done by using a tandem mass correlation algorithm with a mass tolerance of 2.0 Da, to determine whether significant similarities exist between peptides from the tryptic digest and the reconstructed theoretical spectra for a protein in the National Center for Biotechnology Information nonredundant database. Further information about this procedure can be obtained at http://info.med.yale.edu/wmkeck/prochem.htm#ms/mspi.
Assays for Cytolysis of Melanoma Cells by NK Cells and Complement.
The Calcein-acetylmethylester (Calcein-AM) retention procedure (20) was used for both assays. The target cells, either human melanoma lines or primary human fibroblast cells, were isolated from culture flasks in a nonenzymatic dissociation medium (Sigma) and added to 96-well plates (2 × 104 cells/well). The adherent target cells were washed once with PBS and afterward were incubated for 20 min at 37°C in serum-free culture medium (GIBCO/BRL) containing an immunoconjugate (1 μg/ml) or without an immunoconjugate as a control. The target cells then were labeled with 7 μM Calcein-AM (Molecular Probes) in serum-free medium for 40 min at 37°C. Calcein-AM is a fluorescent dye that enters the cells, where it is enzymatically altered and remains intracellular until the cells are lysed. For the cytolytic assays involving NK cells, the labeled target cells were incubated 3–4 hr at 37°C with human NK cells by using the indicated ratios of NK cells to target cells. For the cytolytic assays involving complement, the labeled target cells were incubated 1 hr at 37°C with human serum or purified rabbit complement components (Cedarlane Laboratories). After incubation with NK cells or complement, the target cells were washed twice with PBS, and the fluorescence in the remaining adherent cells (residual fluorescence) was measured with a plate reader. The maximum attainable cytolysis of the target cells was determined by measuring residual fluorescence of the target cells after treatment with lysis buffer (50 mM sodium borate/0.1% Triton X-100, pH 9.0) for 3 hr at 37°C. The maximum residual fluorescence was determined by measuring the fluorescence of adherent target cells that were not exposed to NK cells or complement. The % cytolysis for each sample of target cells was calculated as follows: (residual fluorescence of the sample target cells − residual fluorescence of the lysed target cells)/(maximum residual fluorescence of the target cells − residual fluorescence of the lysed target cells).
RESULTS
Synthesis and Characterization of the Antimelanoma Immunoconjugates.
The immunoconjugate encoded a human IgG1 leader for secretion, a human scFv domain for targeting melanoma cells, and a human IgG1-Fc effector domain (Fig. 1). The immunoconjugate molecules were expressed in transfected CHO and Drosophila S2 cells and were purified from the culture medium as two-chain molecules linked by disulfide bridges in the hinge region of the Fc domain (Figs. 1 and 2). Two immunoconjugates were synthesized, each containing a scFv targeting domain derived from the fusion-phage clone E26–1 or G71–1 (7). The binding specificities of the immunoconjugates E26–1 and G71–1 were tested by cell sorting (FACS) using two human melanoma lines; the controls were primary cultures of normal human melanocyte, fibroblast, microvascular, and umbilical vascular endothelial cells, and the human kidney line 293-EBNA. The results (Fig. 3) show that the immunoconjugates bind strongly to the melanoma cell lines but do not bind to the controls, consistent with the results obtained with the E26–1 and G71–1 fusion-phage clones (7). The binding to melanoma cells occurred when the cells were collected from the culture flask using a nonenzymatic cell dissociation medium (Sigma), but not when the dissociation medium contained trypsin or when the dissociated cells subsequently were treated with trypsin (data not shown). This finding indicates that the cognate melanoma antigen(s) for the immunoconjugates is located on the surface of melanoma cells. The antigen(s) appears to be exceptionally sensitive to trypsin, because the same exposure to trypsin did not affect the binding to the melanoma cells of antibodies against the cell surface molecules intercellular adhesion molecule-1, major histocompatibility complex class I, and tissue factor (data not shown).
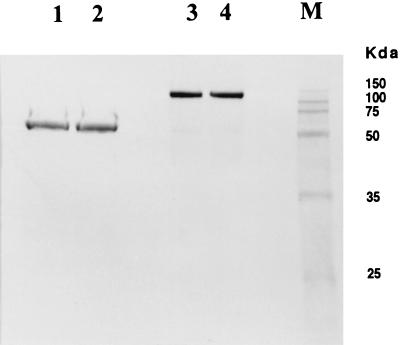
Figure 2.
Anlysis of the immunoconjugate G71–1 by PAGE under reducing (lanes 1 and 2) and nonreducing (lanes 3 and 4) conditions. The immunoconjugate was espressed in CHO cells for lanes 1 and 3, and in Drosophila S2 cells for lanes 2 and 4.
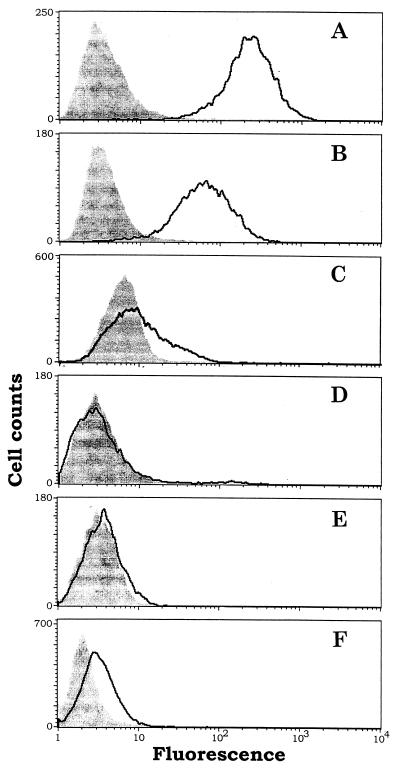
Figure 3.
FACS assays for binding of the immunoconjugate G71–1 to human melanoma cells and human control cells. (A) Melanoma lines, TF2. (B) Melanoma line A-2058. (C) Melanocytes. (D) Microvascular endothelial cells. (E) Fibroblast cells. (F) Transformed kidney line 293-EBNA. The cells were collected from a culture flask after detachment in a nonenzymatic dissociation medium (Sigma). The detached cells were either exposed to the immunoconjugate (outlined curve) or were not exposed (shaded curve). An increase in fluorescence after exposure to the immunoconjugate indicates that the cells bind the immunoconjugate. The number of gated events analyzed for each sample was at least 5,000. The immunoconjugate E26–1 also was analyzed by FACS and showed similar results as G71–1.
Identification of the Cognate Melanoma Antigen for the Immunoconjugates.
Cultured cells from the human melanoma line A2058 were equilibrated with the immunoconjugates G71–1 or E26–1, and the cells were lysed with detergent. The immunoconjugate-antigen complex in the lysate was collected on protein-G beads and analyzed by PAGE. A protein band with an apparent molecular mass of 250 kDa was detected in the melanoma cells but not in the control (Fig. 4). Analysis of a tryptic digest of the protein band by the matrix-assisted laser desorption ionization-MS procedure (17, 18) identified 75 peptide masses that were not present in a digest of a control gel slice. A search of protein sequence databases by profound yielded 50 peptide masses that matched peptide masses in the MCSP core protein (11), spanning 26% of the complete protein sequence. The profound probability score for this identification was 1.0, and the next closest score was 2.2E-61. A search by peptide search matched 45 peptides to the MCSP core protein, with 38 peptides representing the next closest match.
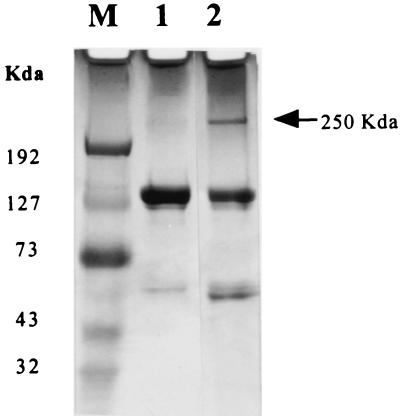
Figure 4.
Immunoprecipitation of a human melanoma protein by immunoconjugate G71–1. The human melanoma line A2058 was equilibrated with the immunoconjugate and lysed with detergent, and the immunoconjugate-protein complex was isolated on protein-G beads and analyzed by PAGE using a 4–15% gradient gel. Lane M, Protein molecular weight markers. Lane 1, immunoconjugate alone. Lane 2, immunoconjugate with associated melanoma protein. The same experiment was done with immunoconjugate E26–1, which also immunoprecipitated a single protein with a molecular mass of 250 kDa.
The tryptic digest of the 250-kDa protein also was analyzed by the liquid chromatography/MS/MS procedure (19). A sequest search of the MS/MS data from the tryptic digest showed significant similarity between the MS/MS spectra for two or more peptides and the reconstructed theoretical MS/MS spectra for two or more peptides from the MCSP core protein in the National Center for Biotechnology Information nonredundant database.
The results of both MS analyses indicate that the melanoma protein immunoprecipitated by the immunoconjugates G71–1 and E26–1 matches the MCSP core protein.
Immunoconjugate-Dependent Cytolysis of Melanoma Cells Mediated by NK Cells and Complement.
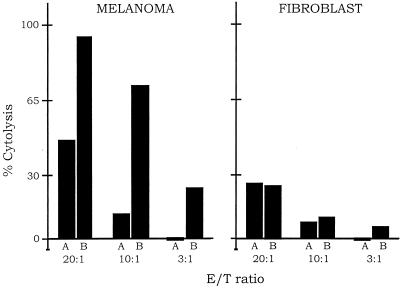
One of the cytolytic pathways of the immune system involves NK cells that can bind directly to target cells, causing antibody-independent lysis of the target cells (21). NK cells also bind to the Fc effector domain of an antibody, resulting in antibody-dependent lysis of cells that bind to the targeting domain of the antibody. This antibody-dependent cell-mediated cytolytic pathway (ADCC) also should cause lysis of cells that bind to the targeting domain of immunoconjugates containing an Fc effector domain. To test for an ADCC response dependent on the immunoconjugates E26–1 and G71–1, melanoma cells and fibroblast control cells were labeled with the fluorescent dye Calcein-AM, and the labeled cells were incubated with human NK cells alone or together with an immunoconjugate. Cytolysis was assayed by measuring the amount of fluorescent dye retained in the cells that remained intact. The results for E26–1 (Fig. 5) show that after incubation with the immunoconjugate and NK cells, the percentage of lysed melanoma cells increased above the basal level that occurs without the immunoconjugate, reaching almost 100% lysis at a 20:1 ratio of NK cells to melanoma cells. In contrast to the efficient lysis of melanoma cells, the fibroblast cells showed no significant increase in cell lysis after incubation with the immunoconjugate and NK cells. Similar results were obtained with the G71–1 immunoconjugate.
Figure 5.
Immunoconjugate-dependent lysis of melanoma cells by NK cells. The melanoma cell line A-2058 and the fibroblast cell control were labeled with the fluorescent dye Calcein-AM. The fraction of melanoma or fibroblast cells remaining intact after exposure to NK cells alone (A bars), or to NK cells with the immunoconjugate E26–1 (B bars), was measured by residual fluorescence. The ratio of NK effector cells to target cells (E/T) was varied from 3 to 20. Three complete sets of experiments were done for both the melanoma and fibroblast cells; for each experiment, the cytolysis assays were done in quadruplicate. The bars represent the average of the cytolysis assays for the three experiments, which generally agreed within 10%. The % cytolysis was calculated as described in Materials and Methods. Similar results were obtained with the immunoconjugate G71–1.
The sensitivity of target cells to lysis by NK cells is increased by expression on the target cell surface of adhesion molecules such as intercellular adhesion molecules (ICAMs) and is reduced by expression of major histocompatibility complex (MHC) class I molecules (22, 23). To determine whether differences in expression of these molecules might contribute to the differences in the sensitivities of melanoma cells and fibroblast cells in the antibody-dependent cell-mediated cytolytic pathway assays, the expression of ICAM-1 and MHC class I molecules by melanoma and fibroblast cells was measured by FACS. Expression of both molecules was similar in the two cell types (data not shown), indicating that the specific lysis of melanoma cells by NK cells depends on the binding of the immunoconjugates to the cognate antigens expressed on melanoma cells.
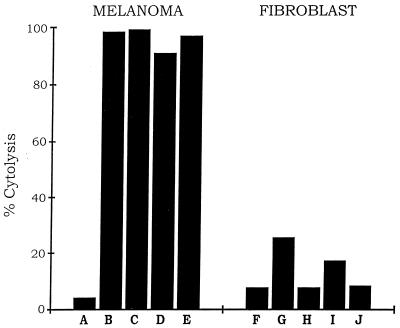
Another cytolytic pathway of the immune system involves the complement cascade, which is activated when the molecule C1q reacts with the Fc region of antibodies bound to a target cell (4). To test for a complement-mediated cytolytic response against melanoma cells, dependent on the immunoconjugates E26–1 and G71–1, the same assay procedure used for the NK-mediated cytolytic response reported above was used with one change, namely that human serum or rabbit serum, which contain the components of the complement cascade, was substituted for NK cells. The results (Fig. 6 and Table 1) show that after incubation with the immunoconjugates and either human serum or rabbit serum, there was an increase in the fraction of melanoma cells lysed from 4% to almost 100%. In contrast to the efficient lysis of melanoma cells, the fibroblast cells showed a small increase in the fraction of lysed cells after incubation with the immunoconjugates and human serum and no significant increase after incubation with the immunoconjugates and rabbit serum.
Figure 6.
Immunoconjugate-dependent lysis of melanoma cells by complement. The procedure was as described in Fig. 5, except that human serum or purified rabbit complement components were used instead of NK cells. Immunoconjugate and complement reagents for each assay were as shown in Table 1.
Table 1.
Immunoconjugate and complement reagents used for each assay in Fig. 6
| Assay | Human serum | Rabbit complement | Immunoconjugate
|
|
|---|---|---|---|---|
| G71-1 | E26-1 | |||
| A, F | − | + | − | − |
| B, G | + | − | + | − |
| C, H | − | + | + | − |
| D, I | + | − | − | + |
| E, J | − | + | − | + |
DISCUSSION
In an earlier study we described the isolation and characterization of several scFv fusion-phage clones, derived from the antibody repertoire of a vaccinated melanoma patient, which bound to human melanoma cells but not to human melanocytes or several other types of normal cells and tumor cells, and therefore appeared to be melanoma specific (7). For the present study the scFv molecules from two of the clones were used as the targeting domains for constructing immunoconjugates containing a human IgG1-Fc effector domain (Fig. 1). The protein immunoprecipitated from human melanoma cells by both immunoconjugates was identified by mass spectrometric analyses as the core protein of a MCSP (9, 10). The MCSP molecule was first identified as the cognate antigen recognized by the mAb 9.2.27 (9), and it appears to be the cognate antigen for several other mAbs (13, 24, 25). Several scFv fusion-phage clones that bind to the melanoma antigen HMW-MAA, which probably is the same as MCSP, have been isolated from a synthetic human scFv library by panning against purified HMW-MAA (26). The MCSP molecule is expressed predominately on the surface of most human melanoma cells (9–13) and also on capillary endothelial cells of glial tumors (27). The finding that MCSP is the cognate antigen for at least two of the melanoma-specific clones isolated from a melanoma patient’s scFv fusion-phage library by panning against melanoma cells (7) suggests that MCSP is a dominant melanoma antigen in vivo.
As an initial test of the therapeutic potential of the two immunoconjugates, an in vitro cytotolytic assay involving fluorescent-labeled target cells was used to determine the capacity of the immunoconjugates to target human melanoma cells for lysis by NK cells and by complement. Both of the immunoconjugates produced a sharp increase in the cytolytic activity of NK cells and complement against melanoma cells, resulting in virtually complete lysis of the targeted melanoma cell population and only minor or no increase in lysis of the fibroblast cells used as a control. There was also a significant background of immunoconjugate-independent cytolysis of melanoma cells and fibroblast cells by NK cells and complement, which is expected for an allogenic assay in which the tumor cells, NK cells, and complement are isolated from different individuals (28). This background should be reduced in a cancer patient, because all of these components are autologous.
The results of the in vitro cytolytic tests provide preliminary evidence that immunoconjugates could have a potential role in immunotherapy protocols with melanoma and other cancers for which tumor-specific scFv or VH (heavy chain variable region) targeting domains are available. It remains to be determined whether such immunoconjugates also can activate a cytolytic immune response in vivo that is sufficiently strong to cause tumor regression and sufficiently specific to avoid unacceptable damage to normal tissues. A limited phase-I clinical trial for melanoma immunotherapy with the mouse mAb 9.2.27, which binds to MCSP, showed specific localization of the antibody in the tumors without evidence of associated toxicity (29). The scFv immunoconjugates that bind to MCSP should be more effective than a mouse mAb for immunotherapy, because the smaller molecular size should improve tumor penetration, and the human derivation of the molecule should minimize an immune rejection response. The G71–1 and E26–1 immunoconjugates probably bind to different MCSP epitopes, as suggested by major differences in their VH sequences (7), and therefore could be administered together to enhance therapeutic efficacy.
Acknowledgments
The experiments involving synthesis of the immunoconjugates and identification of the cognate melanoma antigen were done by B.W., Y.-B.C., and A.G., supported by a gift from private donors (S.L. Misrock and A.M. Fox). The cytolytic assays were done by O.A. and J.B., supported by National Institutes of Health RO1 Grant HL43331. The valuable assistance of Dr. Ying Sun is gratefully acknowledged. The mass spectrometic analyses were done by the Biotechnology Resource Laboratory, W.M. Keck Foundation, Yale University.
ABBREVIATIONS
- scFv
single-chain Fv molecule
- FACS
fluorescence-activated cell sorting
- NK
natural killer
- Calcein-AM
Calcein-acetylmethylester
- CHO
Chinese hamster ovary
- MCSP
melanoma-associated chondroitin sulfate proteoglycan
- MS
mass spectrometry
References
- 1.Bird R E, Walker B W. Trends Biotechnol. 1991;9:132–137. doi: 10.1016/0167-7799(91)90044-i. [DOI] [PubMed] [Google Scholar]
- 2.Vitetta E S, Thorpe P E, Uhr J W. Immunol Today. 1993;14:222–259. doi: 10.1016/0167-5699(93)90041-I. [DOI] [PubMed] [Google Scholar]
- 3.Bruggemann M, Williams G T, Bindon C I, Clark M R, Walker M R, Jefferies R, Waldman H, Neuberger M S. J Exp Med. 1987;166:1351–1361. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fanger M W, Guyre P M. Trends Biotechnol. 1991;9:375–380. doi: 10.1016/0167-7799(91)90129-6. [DOI] [PubMed] [Google Scholar]
- 5.Hansson J, Ohlsson L, Persson R, Andersson G, Ilbäck N-G, Litton M J, Kalland T, Dohlsten M. Proc Natl Acad Sci USA. 1997;94:2489–2494. doi: 10.1073/pnas.94.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Wahab Z A, Weltz C, Hester D, Pickett N, Vervaert C, Barber J R, Jolly D, Seigler H F. Cancer. 1994;80:171–179. doi: 10.1002/(sici)1097-0142(19970801)80:3<401::aid-cncr8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.Cai X, Garen A. Proc Natl Acad Sci USA. 1997;94:9261–9266. doi: 10.1073/pnas.94.17.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamers-Casterman C, Atarhouch T, Muyidermans S, Robinson G, Hamers C, Songa E B, Bendahman N, Hamers R. Nature (London) 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 9.Bumol T F, Reisfeld R A. Proc Natl Acad Sci USA. 1982;79:1245–1249. doi: 10.1073/pnas.79.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bumol T, Walker L E, Reisfeld R A. J Biol Chem. 1984;259:12733–12741. [PubMed] [Google Scholar]
- 11.Pluschke G, Miroslava V, Evans A, Dittmar T, Schmid P, Itin P, Filardo E J, Reisfeld R A. Proc Natl Acad Sci USA. 1996;93:9710–9715. doi: 10.1073/pnas.93.18.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garin-Chesa P, Beresford H R, Carrato-Mena A, Oettgen H F, Old L J, Melamed M R, Rettig W J. Am J Pathol. 1989;134:295–303. [PMC free article] [PubMed] [Google Scholar]
- 13.Reisfeld R A, Cheresh D A. Adv Immunol. 1987;40:323–377. doi: 10.1016/s0065-2776(08)60242-4. [DOI] [PubMed] [Google Scholar]
- 14.Bromberg M E, Konigsberg W H, Madison J F, Pawashe A, Garen A. Proc Natl Acad Sci USA. 1995;92:8205–8209. doi: 10.1073/pnas.92.18.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bender J R, Pardi R, Karasek M A, Engleman E G. J Clin Invest. 1987;79:1679–1688. doi: 10.1172/JCI113007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfau S, Leitenberg D, Rinder H, Smith B R, Pardi R, Bender J R. J Cell Biol. 1995;182:969–978. doi: 10.1083/jcb.128.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams K R, Samandar S M, Stone K L, Saylor M, Rush J. In: The Protein Ptotocols Handbook. Walker J M, editor. Totowa, NJ: Humana; 1996. pp. 541–555. [Google Scholar]
- 18.Mann M, Højrup P, Roepstorff P. Biomed Mass Spectrom. 1993;22:338–345. doi: 10.1002/bms.1200220605. [DOI] [PubMed] [Google Scholar]
- 19.Stone K L, DeAngelis R, LoPresti M, Jones J, Papov V V, Williams K R. Electrophoresis. 1998;19:1046–1052. doi: 10.1002/elps.1150190620. [DOI] [PubMed] [Google Scholar]
- 20.Ayalon O, Hughes E A, Cresswell P, Lee J, O’Donnell L, Pardi R, Bender J R. Proc Natl Acad Sci USA. 1998;95:2435–2440. doi: 10.1073/pnas.95.5.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berke G. Curr Opin Hematol. 1997;4:32–40. doi: 10.1097/00062752-199704010-00006. [DOI] [PubMed] [Google Scholar]
- 22.Zami L, Zauli G, Bavelloni A, Marmiroli S, Cataldi A, Weber G, Vitale M. Cell Immunol. 1995;164:100–104. doi: 10.1006/cimm.1995.1147. [DOI] [PubMed] [Google Scholar]
- 23.Storkus W J, Alexander J, Payne A J, Dawson J R, Cresswell P. Proc Natl Acad Sci USA. 1989;86:2361–2364. doi: 10.1073/pnas.86.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson B S, Imai K, Natali P G, Ferrone S. Int J Cancer. 1981;28:293–300. doi: 10.1002/ijc.2910280307. [DOI] [PubMed] [Google Scholar]
- 25.Hellström I, Garrigues H J, Cabasco L, Mosely G H, Brown J P, Helltröm K E. J Immunol. 1983;130:1467–1472. [PubMed] [Google Scholar]
- 26.Desai S A, Wang X, Noronha E J, Kageshita T, Ferrone S. Cancer Res. 1998;58:2417–2425. [PubMed] [Google Scholar]
- 27.Schrappe M, Kier F G, Spiro R C, Waltz T A, Reisfeld R A, Gladson C L. Cancer Res. 1991;51:4986–4993. [PubMed] [Google Scholar]
- 28.Ciccone E, Pende D, Viale O, Danoto C, Tripodi G, Orengo A, Guardiola J, Moretta A, Moretta L. J Exp Med. 1992;175:709–718. doi: 10.1084/jem.175.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oldham R K, Foon K A, Morgan C, Woodhouse C S, Schroff R W, Abrams P G, Fer M, Schoenberger C S, Farrell M, Kimball E, Sherwin S A. J Clin Oncol. 1984;2:1235–1244. doi: 10.1200/JCO.1984.2.11.1235. [DOI] [PubMed] [Google Scholar]