Abstract
Objective:
We examined whether muscle response times and activation patterns in the lower extremity differed between men and women in response to a rotational knee perturbation while standing in a single-leg, weight-bearing stance.
Design and Setting:
We used a lower extremity perturbation device to produce a sudden, forward, and either internal or external rotation moment of the trunk and femur relative to the weight-bearing tibia. Subjects completed 10 trials of both internal and external rotation perturbation; the first 5 acceptable trials were averaged and used for data analysis. Two separate, repeated-measures analyses of variance were used to compare myoelectric response times and activation patterns between men and women for both internal and external rotation perturbation.
Subjects:
Thirty-two female (19 lacrosse, 13 soccer) and 32 male (lacrosse) healthy intercollegiate athletes participated in the study.
Measurements:
We used surface electromyography to record long latency reflex times of the medial and lateral quadriceps, hamstring, and gastrocnemius muscles.
Results:
Women responded faster than men, primarily due to a shorter latency in quadriceps activation. However, men and women exhibited no difference in muscle-recruitment order.
Conclusions:
Although men and women demonstrated similar muscle-recruitment patterns to an imposed lower extremity perturbation, women tended to activate their quadriceps earlier than men. Whether this earlier quadriceps activation diminishes the ability of the hamstrings to adequately stabilize the knee joint or subjects the anterior cruciate ligament to greater risk of injury is still unknown and requires further study. Furthermore, although surface electromyography and measurement of myoelectric response times are useful in evaluating the timing, activation order, and coactivity patterns of the knee musculature, future studies should evaluate sex differences across the complete response continuum, including measures of intrinsic muscle stiffness, electromechanical delay, and time to force production.
Keywords: electromyography, long latency reflex, anterior cruciate ligament
The higher incidence of anterior cruciate ligament (ACL) injury in female athletes is well known, particularly in sports such as basketball and soccer that require running, cutting, jumping, and sudden deceleration. Although many investigators have attempted to identify potential predisposing factors to explain the higher rate of injuries in women, research to date has failed to conclusively demonstrate the relationship of any 1 variable to ACL injury risk. To address this issue, the American Orthopaedic Society for Sports Medicine, the Orthopaedic Research and Education Foundation, the National Collegiate Athletic Association, and the National Athletic Trainers' Association Research and Education Foundation cosponsored a scientific workshop on noncontact ACL injuries on June 10, 1999, in Hunt Valley, MD.1 Physicians, biomechanists, and certified athletic trainers with expertise in ACL research were brought together to form a scientifically based consensus on what is known regarding ACL injury risk factors and to provide direction for future research. Of the 4 general categories of ACL risk factors considered (anatomical, environmental, hormonal, and biomechanical), the group consensus was that sex differences in neuromuscular and biomechanical function may be the most compelling factors to explain the different rates of injury in men and women.
Considerable attention in recent years has focused on how the muscles about the knee respond when the joint is subject to stress. Failure of the ACL occurs when large mechanical forces are imposed on the knee joint during dynamic activity and exceed the capacity of the stabilizing structures.2 Although the ACL provides most of the static restraint to anterior tibial translation,3 the joint forces incurred in sport go well beyond the capacity of the static restraints, requiring the assistance of active muscle forces to maintain joint equilibrium.2,4,5 In particular, timely activation of the hamstring muscles can assist in protecting the ACL from mechanical strain by stabilizing the tibia, thus reducing anterior and rotary tibial translation.6,7
Efficient neuromuscular control is essential to dynamic joint stiffening and protective stabilization.2,6–13 Neuromuscular control of knee stability is provided through both intentional (preparatory) and reactive (reflexive) responses that are mediated by proprioceptive feed-forward and feedback mechanisms, respectively.10,14 Specific neuromuscular factors that contribute to knee stability include active muscle stiffness15–17 and reflexive muscular activation (reflex latency and electromechanical delay).10,18–22 Furthermore, muscle coactivity6–8,11,12,23 and recruitment patterns23–25 also influence knee stability. Hence, any factor that delays or inhibits 1 or more of these neuromuscular factors compromises knee stability and increases the risk of ACL injury.
The presence of any difference in muscular response characteristics between men and women could be a significant finding in our assessment of neuromuscular factors that may influence ACL injury risk in the female athlete. To date, very few studies have evaluated sex differences in neuromuscular response characteristics.24,26,27 Bell and Jacobs26 and Winter and Brookes27 found no differences in myoelectric response times for reactive elbow flexion and ankle plantar flexion, respectively, but did find the time delay between neural activation and tension development (electromechanical delay) significantly longer in women compared with men. However, these studies evaluated voluntary rather than reflex response times, and whether similar responses exist at the knee is unknown.
Huston and Wojtys24 appeared to be the first to comprehensively and specifically address sex differences in reflex response times at the knee. Using an anterior tibial translation device first described by Wojtys and Huston,25 they evaluated reflex response times and recruitment patterns in men and women after an unanticipated, anteriorly directed force applied to the tibia. Although they found no significant differences in muscle reaction times, female athletes tended to recruit their quadriceps first more often than male athletes or nonathlete controls, who more often recruited their hamstrings first in response to the perturbation. Although their work is significant in that it appears to be the first to specifically address sex differences at the knee, the type of sport and the training of the female athletes (basketball, field hockey, gymnastics, and volleyball athletes) were quite different from those of the male athletes (football) recruited for the study. Other research by these authors5,28 indicated that the type of training (isotonic versus isokinetic versus agility) and level of training (ie, fatigue) can also influence neuromuscular response times. Hence, the difference in skill and training background of the 2 athlete groups may have introduced a potential confounding variable in their assessment of sex differences. Further research addressing sex differences at the knee while controlling for sport and training background is needed.
From the limited research available, it is apparent that more research is needed to determine the influence of sex on neuromuscular response characteristics at the knee, and, thus, their potential role in ACL injury risk. Additionally, we need to assess these differences in a functional manner that is consistent with the muscular conditions and joint positions under which the knee is functioning when these injuries occur.29 In previous studies evaluating reflexive neuromuscular responses at the knee, the perturbation has typically been applied to the joint with the muscles at rest and the lower extremity in a non–weight-bearing or partial weight-bearing position, which does not mimic most injury situations.2,8,25,30–32 Using a functional, weight-bearing perturbation model, we29 have demonstrated that neuromuscular response times and activation patterns are, in fact, quite different from those previously reported under seated, partial weight-bearing, or resting conditions. Therefore, our objective was to determine whether muscle response times and activation patterns in the lower extremity muscles differed between healthy men and women in response to a functional lower extremity perturbation. Our specific aim was to determine whether responses were slower in women than in men, particularly hamstring responses when standing in a single-leg, weight-bearing stance with the muscles actively contracting before the perturbation.
METHODS
Subjects
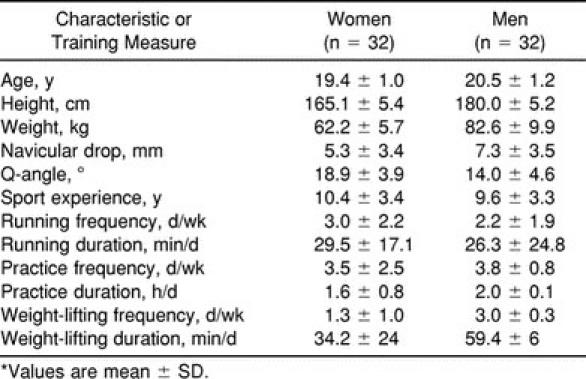
Thirty-two female (19 lacrosse, 13 soccer) and 32 male (lacrosse) healthy intercollegiate athletes participated in the study. These subjects represent the same data set previously reported in Shultz et al.29 Means and standard deviations for subject physical characteristics are listed in Table 1. Before participating in the study, all subjects read and signed an informed consent form approved by the University's Institutional Review Board for the Protection of Human Subjects, which also approved the study.
Table 1.
Physical Characteristics and Training History*
In order to maximize our ability to detect sex differences in neuromuscular response and activation patterns, subjects were matched by type of sport and level of training. Specifically, the sports of intercollegiate soccer and lacrosse were chosen for a number of reasons: (1) They provided a large enough population for adequate statistical power, (2) The 2 sports are similar in their lower extremity performance (ie, similar running and cutting maneuvers on a grass or turf terrain with a cleated shoe), and (3) They are sports in which ACL injuries are likely to occur.
To verify that sport experience and training history were similar between the sexes, data on sport activity and training history were collected on each subject, including running, practice, and weight-lifting activities of the previous month. At the time of this study, lacrosse athletes were in their fall, or nontraditional, season and soccer athletes were in their competitive season. Table 1 indicates that men and women were closely matched in their years of sport experience and current practice activities (both frequency and duration). However, they did differ in their running (frequency) and weight-lifting (frequency and duration) activities in the month before testing. Therefore, although the type and level of sport training and experience were well controlled, current strength and conditioning practices were somewhat variable between the sexes at the time of data collection.
Instrumentation
We used an 8-channel Myosystem 2000 electromyography (EMG) unit (Noraxon, Scottsdale, AZ) and bipolar Ag-AgCl surface electrodes measuring 10 mm in diameter with a center-to-center distance of 2 cm to record myoelectric response times and activation patterns immediately after lower extremity perturbation. Unit specifications include an amplifier gain of 1 mV/V, frequency bandwidth of 16 to 500 Hz, common mode rejection ratio of 114 dB, input resistance from 20 MΩ to 1 GΩ, and a sampling rate of 1000 Hz. To acquire, store, and analyze the EMG data, we used a Gateway 2000 computer (386 processor, AD-16/330, North Sioux City, SD) and Data Pac III Version 1.1 Lab Application Systems software (Run Technologies, Laguna Hills, CA).
We used a lower extremity perturbation device (LEPD) (University of Virginia, Charlottesville, VA) to produce a sudden, forward, and either internal rotation (IR) or external rotation (ER) moment of the trunk and femur relative to the single-leg, weight-bearing tibia. A detailed description of this device and evidence of its reliability and validity has been previously reported.29 We also used an electrogoniometer (Penny and Giles, Santa Monica, CA) and the Chattecx Balance System (Chattanooga Group, Inc, Hixson, TN) to standardize the subject's position before cable release.
Procedures
We prepared the subject's skin and placed surface electrodes in a parallel arrangement over the midline of the vastus lateralis and vastus medialis muscles (midway between the motor point and the distal tendon), medial and lateral hamstring muscles (mid-belly position), and medial and lateral gastrocnemius muscles (mid-belly position). We then placed the electrogoniometer across the lateral aspect of the knee joint and secured both electrodes and electrogoniometer with an elastic bandage to prevent cable tensioning and movement artifact. To position the subject in the LEPD, we adjusted the waist harness to fit snugly at the level of the anterior superior iliac spine and the height of the wall-mounted trigger mechanism to a position level with the anterior superior iliac spine while the subject was in the test position.
Standing on the Chattecx Balance System platform, with cables attached and height properly adjusted at the wall, each subject was instructed to stand upright in a single-leg stance with back straight and arms folded across the chest and to lean into the cables with the hips. From this position, we asked the subject to slowly bend the knee to a knee flexion angle of 35°, as indicated by the electrogoniometer. Using the Chattecx's visual training target, we moved the footplate vertically on the platform until the cursor was located in the middle of the target (bull's eye), indicating that the center of pressure was being directed through the middle of the subject's foot.
Once the subject was properly positioned, we released either the left or right cable to produce either IR or ER perturbation. We instructed the subject to look straight ahead and to react to the perturbation by attempting to maintain the single-leg balance. We allowed each subject a minimum of 3 practice trials for both IR and ER perturbations to become acquainted and comfortable with the task. Each subject then underwent 10 trials of both IR and ER perturbation, using the dominant leg for all trials. The dominant leg was identified as the foot the subject would use to kick a ball. Subjects rested 30 seconds between trials to prevent fatigue, and the direction of perturbation was randomized to avoid anticipatory responses.
Signal Processing and Analysis
We recorded EMG activity for 100 milliseconds before and 900 milliseconds after lower extremity perturbation using a trigger sweep acquisition mode. We processed the signal with root mean square (10-millisecond time constant) and used 2 threshold event buffers to separate the IR and ER events. We then compiled an averaged signal, using the first 5 acceptable events, to determine the muscle response time for each muscle. Each trial was visually inspected, and an acceptable trial was determined by the following criteria: (1) long latency reflex identified within 150 milliseconds from perturbation onset, (2) baseline muscle activity sufficiently quiet and stable to ensure an acceptable signal-to-noise ratio, (3) readable signal obtained from all 6 muscles, and (4) signal free of movement artifact to allow clear interpretation.29
We defined muscle response time as the time delay between the initiation of the perturbation and the onset of EMG activity at the long latency response level. We chose to evaluate the long latency reflex response because it has been demonstrated to be the first response to consistently occur under active muscular conditions.33,34 Using the 100-millisecond pretrigger as the reference interval, we defined the long latency response as the time point when myoelectric activity exceeded 2 standard deviations of the average baseline activity in the hamstrings and gastrocnemius and 1 standard deviation in the quadriceps within the time interval of 30 to 150 milliseconds.29
We used 2 separate, mixed-design analyses of variance with 1 between-groups (sex) and 2 within-groups (muscle group at 3 levels [quadriceps, hamstring, gastrocnemius], and muscle side at 2 levels [medial, lateral]) to determine if men and women differed in muscle response times for either IR or ER perturbation conditions. We used the Tukey post hoc analysis to evaluate any significant main effects or interactions. We determined muscle activation order using a repeated-measures analysis of variance with 1 between-groups (sex) and 1 within-groups (muscle) factors at 6 levels (lateral quadriceps [LQ], medial quadriceps [MQ], lateral hamstring [LH], medial hamstring [MH], lateral gastrocnemius [LG], and medial gastrocnemius [MG]). We used the Tukey honestly significant difference (HSD) method to evaluate the main effect for muscle as to which muscles differed significantly in their response time. To determine whether men and women differed in their recruitment order, we evaluated the sex-by-muscle interaction. An α level of P < .05 was used for all analyses.
RESULTS
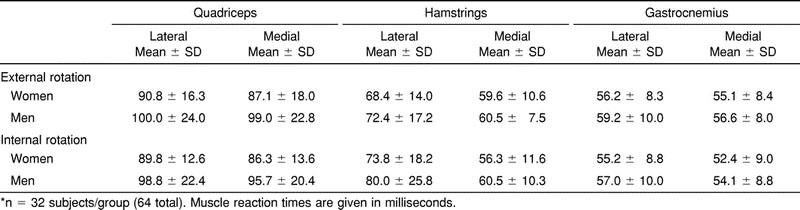
Table 2 lists the group means and standard deviations by sex for the muscle reaction times obtained for both IR and ER perturbation conditions. We found that women responded significantly faster than men for both ER (F1,62 = 5.209, P = .026) and IR (F1,62 = 6.642, P = .012). Although this difference appeared to be due primarily to a shorter latency in quadriceps activation in women, no significant sex-by-muscle group (ER, P = .132; IR, P = .281) or muscle-side (ER, P = .693; IR, P = .771) interactions were found.
Table 2.
Muscle Reaction Times for External Rotation and Internal Rotation Perturbation*
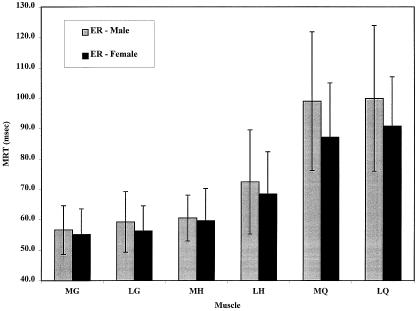
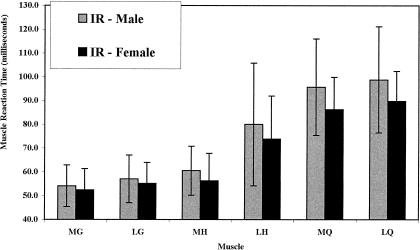
For muscle-recruitment order, we found a preferential muscle-recruitment order for both ER (F5,310 = 120.59, P < .0001) and IR (F5,310 = 107.40, P < .0001). For ER, the Tukey post hoc analysis revealed no difference in response times for the MG (55.8 milliseconds), LG (57.7 milliseconds), and MH (60.0 milliseconds), which were the first muscles to respond to the perturbation. The LH (70.4 milliseconds) responded next after a significant delay, and then the MQ (93.0 milliseconds) and LQ (95.3 milliseconds) were activated following an even greater delay (Figure 1). Our findings were similar for IR (MG [53.2 milliseconds] = LG [56.1 milliseconds] = MH [58.4 milliseconds] < LH [76.9 milliseconds] < MQ [91.0 milliseconds] = LQ [94.3 milliseconds]) (Figure 2). No difference in muscle-recruitment order was found between men and women for either ER (F5,310 = 1.843, P = .104) or IR (F5,310 = .926, P = .464).
Figure 1.
Muscle reaction times for men and women after external rotation (ER) perturbation. Error bars indicate SDs. Lateral hamstring (LH) was significantly slower than medial or lateral gastrocnemius (MG, LG) or medial hamstring (MH). Medial and lateral quadriceps (MQ, LQ) were significantly slower than LH (Tukey, P < .05).
Figure 2.
Muscle reaction times for men and women after internal rotation (IR) perturbation. Error bars indicate SDs. Lateral hamstring (LH) was significantly slower than medial or lateral gastrocnemius (MG, LG) or medial hamstring (MH). Medial and lateral quadriceps (MQ, LQ) were significantly slower than LH (Tukey, P < .05).
DISCUSSION
Our primary finding was that women responded faster than men at the long latency response levels for both IR and ER perturbation conditions. Although we did not find a sex difference in recruitment order for either IR or ER, it is readily apparent that the faster time in women was due primarily to a shorter latency in quadriceps activation (Table 2 and Figures 1 and 2). In fact, had we evaluated each muscle group separately and not corrected for type I error, we would have found a significant sex difference in the quadriceps but no differences in the hamstring and gastrocnemius muscle response times. Thus, it appears our findings were muscle dependent.
Our results indicate a difference of approximately 10 milliseconds in quadriceps activation times between men and women (Table 2). Given the inherent variability associated with EMG recordings and human performance, it is important to first evaluate whether 10 milliseconds represents a true difference between men and women. In a previous article, we addressed the issues of reliability (ie, performance consistency) using this research model.29 We found, using a 2-trial average, that the standard error of measurement for the quadriceps response times averaged 7.6 milliseconds, indicating a 95% confidence interval of ±14.9 milliseconds. However, it is important to note that this estimate of measurement error was based on a 2-trial average, and in this study, we used a 5-trial average in an effort to increase reliability and reduce measurement error. Moreover, this measurement error reflects primarily a random variability in individual subject performance. As such, one would not expect this performance inconsistency to be different between groups, the net result being that this error would only serve to minimize our ability to find a significant difference between men and women. It would not explain why a group of women would perform consistently faster and men consistently slower. So although these data illustrate the variability inherent in EMG studies and human performance measures, we believe the 10-millisecond difference, although modest, is clinically relevant. Given these findings, we considered potential factors that may explain the quadriceps-specific difference.
Decreased Quadriceps Inhibition in Women
After the perturbation, a silent period (ie, reflexive silencing of the muscle) was consistently observed in the medial and lateral quadriceps that was not observed in the other muscle groups. Considering the method of the imposed perturbation, this silent period is likely to be either a function of reflexive inhibition in response to sudden unloading of the contracting quadriceps34–36 or a reciprocal inhibition in response to reflexive activation of the antagonist (hamstring) muscle group.21 Although actual onset times were not calculated for this silent period, they appeared to consistently coincide with activation of the medial hamstring.
Regardless of the mechanism, the long latency reflex activation of the quadriceps typically occurred immediately after this silent period, indicating an earlier onset or shorter duration of inhibition in women than in men. Hence, it is plausible that women may exhibit less quadriceps inhibition compared with men in response to joint loading. However, even though women exhibited a shorter quadriceps delay, quadriceps latency times were still significantly longer than those for the hamstrings and gastrocnemius muscles. Alternately, given the typical strength differential of the quadriceps and hamstrings, a greater delay in quadriceps activation could conceivably provide the weaker hamstrings with more time to stabilize the tibia before an anterior tibial translation force is created. Whether this difference is sufficient to diminish the hamstrings' ability to prevent excessive anterior or rotary tibial translation or subject the ACL to greater risk is unknown.
Preferential Activation
Previous work by Huston and Wojtys24 indicated that female athletes may exhibit a preferential quadriceps activation compared with male athletes. When evaluating individual subjects, they found that female athletes tended to activate the quadriceps first more often than male athletes at the intermediate (ie, long latency reflex) response level when compared with the hamstrings and gastrocnemius muscles after an anteriorly directed tibial translation force. Preferential activation of the quadriceps was rarely seen in the present study, most likely due to the difference in perturbation methods29 and the presence of the reflexive silent period in the quadriceps. In fact, the quadriceps were activated first in only 1 of 64 subjects (a male subject). Only 4 subjects (2 female subjects and 2 male subjects) initiated the quadriceps before the hamstrings. Although this trend was infrequent and did not appear to be sex specific, the activation patterns demonstrated by these subjects clearly deviated from the norm.
Training History
Although we expended considerable effort to control for training variability, the female and male athletes in this study did differ somewhat in their running and weight-lifting activities in the month before data collection. Considering the extent of running and agility activities inherent in practice sessions, one would not expect the additional day of running per week in the female group to be a factor. There was, however, a considerable difference in both the duration and frequency of isotonic weight-lifting activities between the groups. In research evaluating the effects of isotonic strength training on reflex response times, no significant differences have been found in monosynaptic reflex times of the quadriceps after 16 weeks of training37 or in intermediate response times of the quadriceps, hamstrings, or gastrocnemius after 6 weeks of training.28 Therefore, the difference in weight-training practices would not seem to provide a plausible explanation for this sex difference. It is possible that the female group may have engaged in more agility-training activities, which have been shown to decrease response times at both reflex and voluntary levels.28 Unfortunately, sport-specific practice activities were not controlled or documented in this study, and therefore, no conclusions can be made in this regard.
Although these data and this discussion pose more questions than answers, our findings of earlier quadriceps activation in women remain of interest when considering potential ACL injury risk factors. Whether the decreased quadriceps inhibition exhibited by these subjects alters the hamstrings' ability to stabilize the tibia and places the ACL at greater risk is unknown. Furthermore, since training is known to influence neuromuscular response characteristics,5,8,28,38 our findings in intercollegiate soccer and lacrosse athletes may not be representative of other sports (ie, basketball, gymnastics, and volleyball) or skill levels (ie, high school, recreational). Hence, further research investigating the influence of early quadriceps activation on dynamic joint stability and ACL injury risk during functional weight-bearing activities in subjects from a variety of sports and skill levels is warranted.
Clinical Relevance
This study has demonstrated the effective use of surface EMG to determine muscle response times and activation patterns of the lower extremity muscles after a loading stress to the knee. The specific intent of this research was to evaluate reflexive neuromuscular responses under functional weight-bearing conditions, which had not been previously addressed. From this information, the coordinative proprioceptive and neuromuscular mechanisms necessary to protect the joint and prevent ligament injury under functional conditions can be better understood. However, it is well recognized that latency times derived from the EMG signal only reflect the time required for the nerve impulse to reach the muscle fibers, and they are not synonymous with the development of muscle tension.26,27 Specifically, myoelectric response latencies do not provide any sense of tension development in the muscle before reflexive activation (intrinsic stiffness) or reflect the additional time delay required for muscular tension to develop after neural activation (ie, electromechanical delay).
Considering the evidence that suggests that force delays may be longer in women than in men,24,26,27 the fact that women responded faster myoelectrically in this study does not necessarily indicate that they produced muscular tension any sooner than men. Measuring both reflex and force components of the neuromuscular response continuum in future studies will provide a more comprehensive picture of sex-specific neuromuscular response patterns at the knee and their potential influence on musculoskeletal stability and ACL injury risk.
Adequacy of Neuromuscular Reflexes in Joint Protection
Although laboratory experiments have established the effectiveness of active muscles in limiting joint excursion,6,7,11,12 the adequacy of reflexive neuromuscular responses in protecting the joint given the extremely high and rapid force applications imposed on the knee in sports has been called into question.31,39 Pope et al31 suggested through mathematical modeling that reflexive activation occurs after medial collateral ligament failure if the muscles are relaxed at the time of loading and if the ligament-muscle protective reflex is initiated by pain. Konradsen et al39 investigated the role of protective muscular responses in ankle stabilization following a sudden forced inversion and determined the reflexive activation of the peroneal muscles was too slow to prevent excessive inversion and ligament injury. However, these research models did not account for the contribution of active muscle stiffness15–17,22 or preparatory alterations in muscular tension at lower applied loads10,40 that may prevent excessive joint excursion or provide some measure of immediate joint stiffening that augments the reflexive response.
Active muscle stiffness, or mechanical resistance to muscle loading or stretch in a preactivated muscle, provides the most immediate response to limit excessive joint motion and can result in tension development as early as 10 milliseconds.15 Since active muscle stiffness is linearly related to the extent of cross-bridge formation,15–17 the extent of muscle contraction at the time of perturbation would appear to be an important factor in the adequacy of this immediate stiffening response and subsequent joint stabilization until reflexive activation can occur. Thus, to truly evaluate the adequacy of reactive muscular responses in providing joint protection with sudden loading, one must also consider the contribution of active muscle stiffness in situations in which the muscle may be actively contracting before the perturbation or application of the injurious force.
In addition to active muscle stiffness, which joint structure is responsible for the proprioceptive feedback may also influence the adequacy of the neuromuscular response. For example, if the high-threshold Golgi tendon receptors located in ligament structures are the first to be stimulated, it is likely that the initiated muscular responses will not occur quickly enough, given that the delays in force generation would be greater than the time required to reach ligament failure.31,39 In contrast, sensory feedback provided by other joint structures at lower mechanical thresholds may stimulate a stiffening response before excessive ligament loading and effectively prevent joint deformation from reaching the point of ligament failure. In fact, Johansson et al10 and Sojka et al40 provided evidence that low-threshold receptors in the cruciate ligaments can influence the sensitivity of the muscle spindle and provide preparatory stiffening of the muscle before excessive loading.41
Clearly, more research is needed to determine the efficacy of various proprioceptive feedback mechanisms and the ultimate capacity of neuromuscular responses to stiffen the joint and protect the ACL under sudden loading conditions. Thus, determining whether men and women differ in their mechanical or neuromuscular responses remains a clinically relevant question that may explain the disparity in ACL injury rates. Our hope is that this discussion will stimulate more research in this area.
SUMMARY
Determining whether men and women differ in their neuromuscular response characteristics remains a clinically relevant question that may in part explain the sex disparity in ACL injury rates. We specifically investigated whether myoelectric response times differed in male and female athletes after knee perturbation in a functional, weight-bearing position. Our findings indicate that although men and women demonstrated similar muscle-recruitment patterns, women tended to activate their quadriceps earlier than men. Whether this earlier quadriceps activation diminishes the ability of the hamstrings to adequately stabilize the knee joint and subjects the ACL to greater risk of injury is still unknown and requires further study. Future studies should evaluate sex differences across the complete response continuum, including measures of intrinsic muscle stiffness, electromechanical delay, and time to force production, in both healthy and ACL-injured athletes from a variety of sports and skill levels.
ACKNOWLEDGMENTS
We thank Bob Anderson, Laboratory Instrument Maker in the Department of Biomedical Engineering at the University of Virginia, for his assistance with the construction of the Lower Extremity Perturbation Device (LEPD). Funding for the construction of the LEPD was provided through a research grant from the Far West Athletic Trainers Association. This study was funded by the National Athletic Trainers' Association Research and Education Foundation.
REFERENCES
- 1.Griffin LY, Agel J, Albohm M, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Solomonow M, Baratta R, Zhou BH, et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15:207–213. doi: 10.1177/036354658701500302. [DOI] [PubMed] [Google Scholar]
- 3.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee: a biomechanical study. J Bone Joint Surg Am. 1980;62:259–270. [PubMed] [Google Scholar]
- 4.Balestra C, Duchateau J, Hainaut K. Effects of fatigue on the stretch reflex in a human muscle. Electroencephalogr Clin Neurophysiol. 1992;85:46–52. doi: 10.1016/0168-5597(92)90101-g. [DOI] [PubMed] [Google Scholar]
- 5.Wojtys EM, Wylie BB, Huston LJ. The effects of muscle fatigue on neuromuscular function and anterior tibial translation in healthy knees. Am J Sports Med. 1996;24:615–621. doi: 10.1177/036354659602400509. [DOI] [PubMed] [Google Scholar]
- 6.Hirokawa S, Solomonow M, Luo Z, D'Ambrosia R. Muscular co-contraction and control of knee stability. J Electromyogr Kinesiol. 1991;1:199–208. doi: 10.1016/1050-6411(91)90035-4. [DOI] [PubMed] [Google Scholar]
- 7.Renstrom P, Arms SW, Stanwyck TS, Johnson RJ, Pope MH. Strain within the anterior cruciate ligament during hamstring and quadriceps activity. Am J Sports Med. 1986;14:83–87. doi: 10.1177/036354658601400114. [DOI] [PubMed] [Google Scholar]
- 8.Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D'Ambrosia R. Muscular coactivation: the role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16:113–122. doi: 10.1177/036354658801600205. [DOI] [PubMed] [Google Scholar]
- 9.Hagood S, Solomonow M, Baratta R, Zhou BH, D'Ambrosia R. The effect of joint velocity on the contribution of the antagonist musculature to knee stiffness and laxity. Am J Sports Med. 1990;18:182–187. doi: 10.1177/036354659001800212. [DOI] [PubMed] [Google Scholar]
- 10.Johansson H. Role of knee ligaments in proprioception and regulation of muscle stiffness. J Electromyogr Kinesiol. 1991;1:158–179. doi: 10.1016/1050-6411(91)90032-Z. [DOI] [PubMed] [Google Scholar]
- 11.Louie J, Mote C. Contribution of the musculature to rotary laxity and torsional stiffness at the knee. J Biomech. 1987;20:281–300. doi: 10.1016/0021-9290(87)90295-8. [DOI] [PubMed] [Google Scholar]
- 12.Markolf KL, Graff-Radford A, Amstutz HC. In vivo knee stability: a quantitative assessment using an instrumented clinical testing apparatus. J Bone Joint Surg Am. 1978;60:664–674. [PubMed] [Google Scholar]
- 13.Walla DJ, Albright JP, McAuley E, Martin RK, Eldridge V, El-Khoury G. Hamstring control and the unstable anterior cruciate ligament-deficient knee. Am J Sports Med. 1985;13:34–39. doi: 10.1177/036354658501300106. [DOI] [PubMed] [Google Scholar]
- 14.Johansson H, Sjolander P, Sojka P. A sensory role for the cruciate ligaments. Clin Orthop. 1991;268:161–179. [PubMed] [Google Scholar]
- 15.Hoffer JA, Andreassen S. Regulation of soleus muscle stiffness in premammillary cats: intrinsic and reflex components. J Neurophysiol. 1981;45:267–285. doi: 10.1152/jn.1981.45.2.267. [DOI] [PubMed] [Google Scholar]
- 16.Joyce GC, Rack PM. Isotonic lengthening and shortening movements of cat soleus muscle. J Physiol. 1969;204:475–491. doi: 10.1113/jphysiol.1969.sp008925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rack PM, Westbury DR. The short range stiffness of active mammalian muscle and its effect on mechanical properties. J Physiol. 1974;240:331–350. doi: 10.1113/jphysiol.1974.sp010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrack RL, Lund PJ, Skinner HB. Knee joint proprioception revisited. J Sport Rehabil. 1994;3:18–42. [Google Scholar]
- 19.Eccles JC. Physiology of motor control in man. Appl Neurophysiol. 1981;44:5–15. doi: 10.1159/000102178. [DOI] [PubMed] [Google Scholar]
- 20.Enoka RM. Neuromechanical Basis of Kinesiology. 2nd ed. Champaign, IL: Human Kinetics; 1994. [Google Scholar]
- 21.Kandel ER, Schwartz JH, Jessell TM. Essentials of Neural Science. Norwalk, CT: Appleton and Lange; 1995. [Google Scholar]
- 22.Kearney RE, Stein RB, Parameswaran L. Identification of intrinsic and reflex contributions to human ankle stiffness dynamics. IEEE Trans Biomed Eng. 1997;44:493–504. doi: 10.1109/10.581944. [DOI] [PubMed] [Google Scholar]
- 23.Gauffin H, Tropp H. Altered movement and muscular-activation patterns during the one-legged jump in patients with an old anterior cruciate ligament rupture. Am J Sports Med. 1992;20:182–192. doi: 10.1177/036354659202000215. [DOI] [PubMed] [Google Scholar]
- 24.Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24:427–436. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- 25.Wojtys EM, Huston LJ. Neuromuscular performance in normal and anterior cruciate ligament-deficient lower extremities. Am J Sports Med. 1994;22:89–104. doi: 10.1177/036354659402200116. [DOI] [PubMed] [Google Scholar]
- 26.Bell DG, Jacobs I. Electro-mechanical response times and rate of force development in males and females. Med Sci Sports Exerc. 1986;18:31–36. [PubMed] [Google Scholar]
- 27.Winter EM, Brookes FBC. Electromechanical response times and muscle elasticity in men and women. Eur J Appl Physiol Occup Physiol. 1991;63:124–128. doi: 10.1007/BF00235181. [DOI] [PubMed] [Google Scholar]
- 28.Wojtys EM, Huston LJ, Taylor PD, Bastian SD. Neuromuscular adaptations in isokinetic, isotonic, and agility training programs. Am J Sports Med. 1996;24:187–192. doi: 10.1177/036354659602400212. [DOI] [PubMed] [Google Scholar]
- 29.Shultz SJ, Perrin DH, Adams JM, Arnold BL, Gansneder BM, Granata KP. Assessment of neuromuscular response characteristics at the knee following a functional perturbation. J Electromyogr Kinesiol. 2000;10:159–170. doi: 10.1016/s1050-6411(00)00002-x. [DOI] [PubMed] [Google Scholar]
- 30.Beard DJ, Kyberd PJ, Fergusson CM, Dodd CA. Proprioception after rupture of the anterior cruciate ligament: an objective indication of the need for surgery? J Bone Joint Surg Br. 1993;75:311–315. doi: 10.1302/0301-620X.75B2.8444956. [DOI] [PubMed] [Google Scholar]
- 31.Pope MH, Johnson RJ, Brown DW, Tighe C. The role of the musculature in injuries to the medial collateral ligament. J Bone Joint Surg Am. 1979;61:398–402. [PubMed] [Google Scholar]
- 32.Small C, Waters JT, Jr, Voight M. Comparison of two methods for measuring hamstring reaction time using the Kin-Com isokinetic dynamometer. J Orthop Sports Phys Ther. 1994;19:335–340. doi: 10.2519/jospt.1994.19.6.335. [DOI] [PubMed] [Google Scholar]
- 33.Chan CWY, Jones GM, Kearney RE, Watt DGD. The ‘late’ electromyographic response to limb displacement in man, 1: evidence for supraspinal contribution. Electroencephalogr Clin Neurophysiol. 1979;46:173–181. doi: 10.1016/0013-4694(79)90066-x. [DOI] [PubMed] [Google Scholar]
- 34.Marsden CD. Servo control, the stretch reflex and movement in man. In: Desmedt JE, editor. New Developments in Electromyography and Clinical Neurophysiology. Basel, Switzerland: Karger; 1973. pp. 375–382. [Google Scholar]
- 35.Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. 2nd ed. Philadelphia, PA: FA Davis; 1989. [Google Scholar]
- 36.Shahani BT, Young RR. Studies of the normal human silent period. In: Desmedt JE, editor. New Developments in Electromyography and Clinical Neurophysiology. Basel, Switzerland: Karger; 1973. pp. 588–602. [Google Scholar]
- 37.Hakkinen K, Komi PV. Changes in neuromuscular performance in voluntary and reflex contraction during strength training in man. Int J Sports Med. 1983;4:282–288. doi: 10.1055/s-2008-1026051. [DOI] [PubMed] [Google Scholar]
- 38.Wilk KE, Escamilla RF, Fleisig GS, Barrentine SW, Andrews JR, Boyd ML. A comparison of tibiofemoral joint forces and electromyographic activity during open and closed kinetic chain exercises. Am J Sports Med. 1996;24:518–527. doi: 10.1177/036354659602400418. [DOI] [PubMed] [Google Scholar]
- 39.Konradsen L, Voigt M, Hojsgaard C. Ankle inversion injuries: the role of the dynamic defense mechanism. Am J Sports Med. 1997;25:54–58. doi: 10.1177/036354659702500110. [DOI] [PubMed] [Google Scholar]
- 40.Sojka P, Johansson H, Sjolander P, Lorentzon R, Djupsjobacka M. Fusimotor neurones can be reflexly influenced by activity in receptor afferents from the posterior cruciate ligament. Brain Res. 1989;483:177–183. doi: 10.1016/0006-8993(89)90051-6. [DOI] [PubMed] [Google Scholar]
- 41.Johansson H, Sjolander P, Sojka P. Receptors in the knee joint ligaments and their role in the biomechanics of the joint. Crit Rev Biomed Eng. 1991;18:341–368. [PubMed] [Google Scholar]