Abstract
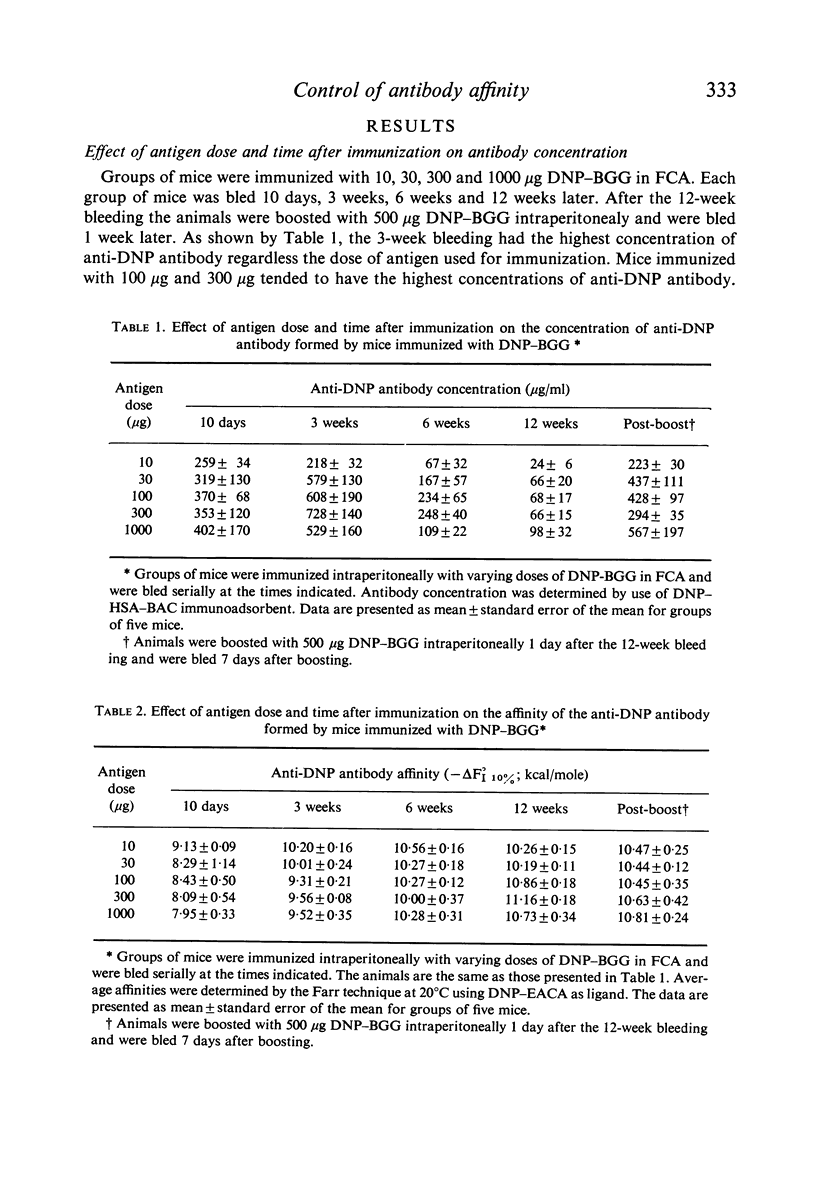
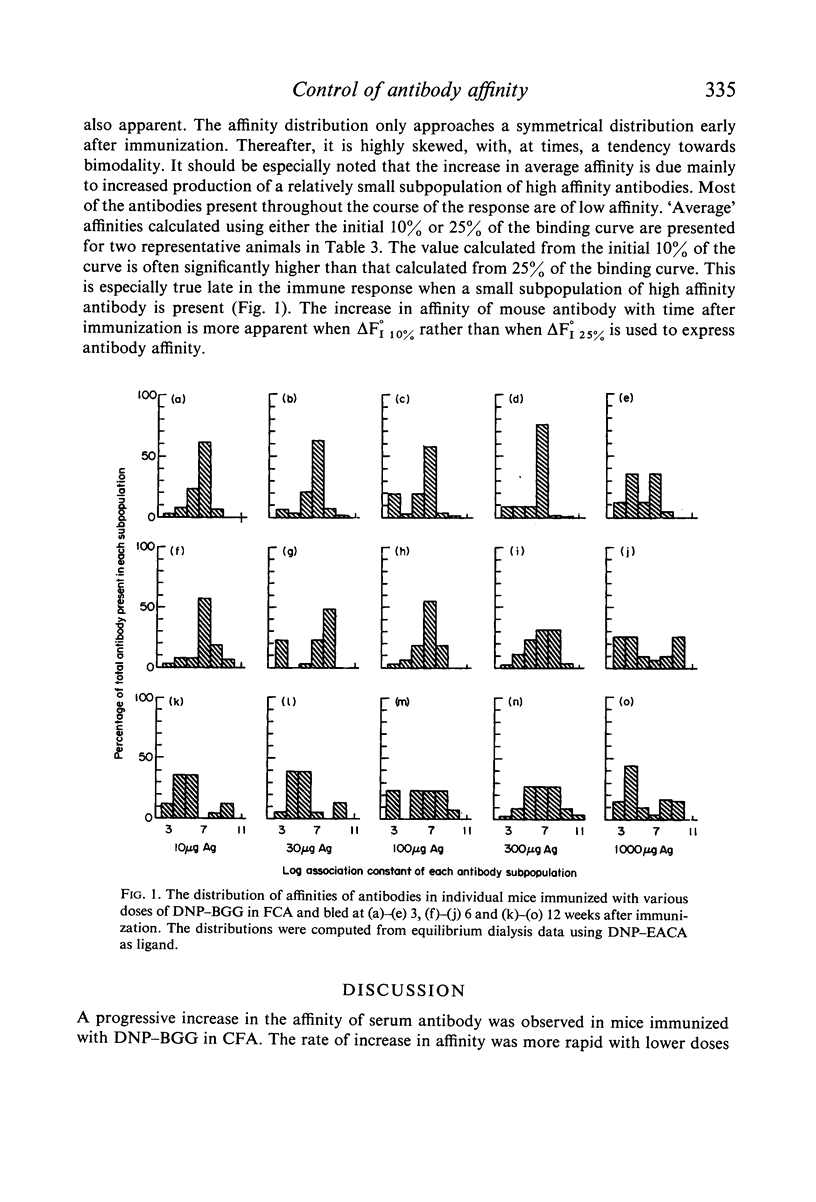
The effect of antigen dose and time after immunization on the affinity of serum antihapten antibody was studied in the mouse by the Farr technique and by equilibrium dialysis. A progressive increase in affinity was seen with time after immunization at all antigen dose levels. The rate of increase in affinity was faster with lower doses of antigen. However, the increase in affinity continued for a longer time in animals immunization with larger doses of antigen. Consequently, at 3 months after immunization animals injected with a larger dose of antigen had higher average affinity antibody than did animals immunized with low doses of antigen. Low affinity antibody was produced in significant amounts at all immunizing dose levels and was present throughout the course of the immune response. Certain technical problems in interpretation of equilibrium dialysis data are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B. Studies on the regulation of avidity at the level of the single antibody-forming cell. The effect of antigen dose and time after immunization. J Exp Med. 1970 Jul 1;132(1):77–88. doi: 10.1084/jem.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Receptors on immunocompetent cells. V. Cellular correlates of the "maturation" of the immune response. J Exp Med. 1972 Mar 1;135(3):660–674. doi: 10.1084/jem.135.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions on Original Antigenic Sin. II. Proof in lower creatures. J Exp Med. 1966 Sep 1;124(3):347–361. doi: 10.1084/jem.124.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio H., Karush F. Antibody affinity. II. Effect of immunization interval on antihapten antibody in the rabbit. Biochemistry. 1966 Jun;5(6):1856–1863. doi: 10.1021/bi00870a011. [DOI] [PubMed] [Google Scholar]
- GREY H. M. STUDIES ON CHANGES IN THE QUALITY OF RABBIT-BOVINE SERUM ALBUMIN ANTIBODY FOLLOWING IMMUNIZATION. Immunology. 1964 Jan;7:82–90. [PMC free article] [PubMed] [Google Scholar]
- Goidl E. A., Paul W. E., Siskind G. W., Benacerraf B. The effect of antigen dose and time after immunization on the amount and affinity of anti-hapten antibody. J Immunol. 1968 Feb;100(2):371–375. [PubMed] [Google Scholar]
- Huchet R., Feldmann M. Studies on antibody affinity in mice. Eur J Immunol. 1973 Jan;3(1):49–55. doi: 10.1002/eji.1830030111. [DOI] [PubMed] [Google Scholar]
- Klinman N. R., Rockey J. H., Frauenberger G., Karush F. Equine anti-hapten antibody. 3. The comparative properties of gamma G- and gammaA-antibodies. J Immunol. 1966 Apr;96(4):587–595. [PubMed] [Google Scholar]
- Klinman N. R., Taylor R. B. General methods for the study of cells and serum during the immune response: the response to dinitrophenyl in mice. Clin Exp Immunol. 1969 Apr;4(4):473–487. [PMC free article] [PubMed] [Google Scholar]
- Lamelin J. P., Paul W. E. Rate of increase of antibody affinity in different rat strains. Int Arch Allergy Appl Immunol. 1971;40(3):351–360. doi: 10.1159/000230418. [DOI] [PubMed] [Google Scholar]
- Miller G. W., Segre D. Determination of relative affinity and heterogeneity of mouse anti-DNP antibodies by a plaque-inhibition technique. J Immunol. 1972 Jul;109(1):74–83. [PubMed] [Google Scholar]
- Parker C. W., Godt S. M., Johnson M. C. Fluorescent probes for the study of the antibody-hapten reaction. II. Variation in te antibody combining site during the immune response. Biochemistry. 1967 Nov;6(11):3417–3427. doi: 10.1021/bi00863a012. [DOI] [PubMed] [Google Scholar]
- Petty R. E., Steward M. W., Soothill J. F. The heterogeneity of antibody affinity in inbred mice and its possible immunopathologic significance. Clin Exp Immunol. 1972 Oct;12(2):231–241. [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., Haimovich J., Sela M. Purification of antibodies with immunoadsorbents prepared using bromoacetyl cellulose. Immunochemistry. 1967 Jan;4(1):11–22. doi: 10.1016/0019-2791(67)90192-9. [DOI] [PubMed] [Google Scholar]
- Sarvas H., Mäkelä O. Haptenated bacteriophage in the assay of antibody quantity and affinity: maturation of an immune response. Immunochemistry. 1970 Nov;7(11):933–943. doi: 10.1016/0019-2791(70)90054-6. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Dunn P., Walker J. G. Studies on the control of antibody synthesis. II. Effect of antigen dose and of suppression by passive antibody on the affinity of antibody synthesized. J Exp Med. 1968 Jan 1;127(1):55–66. doi: 10.1084/jem.127.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda S., Schlamowitz M. Studies of 125I trace labeling of immunoglobulin G by chloramine-T. Immunochemistry. 1970 Nov;7(11):885–898. doi: 10.1016/0019-2791(70)90051-0. [DOI] [PubMed] [Google Scholar]
- Stupp Y., Yoshida T., Paul W. E. Determination of antibody-hapten equilibrium constants by an ammonium sulfate precipitation technique. J Immunol. 1969 Sep;103(3):625–627. [PubMed] [Google Scholar]
- Theis G. A., Siskind G. W. Selection of cell populations in induction of tolerance: affinity of antibody formed in partially tolerant rabbits. J Immunol. 1968 Jan;100(1):138–141. [PubMed] [Google Scholar]
- Werblin T. P., Kim Y. T., Quagliata F., Siskind G. W. Studies on the control of antibody synthesis. 3. Changes in heterogeneity of antibody affinity during the course of the immune response. Immunology. 1973 Mar;24(3):477–492. [PMC free article] [PubMed] [Google Scholar]
- Werblin T. P., Siskind G. W. Distribution of antibody affinities: technique of measurement. Immunochemistry. 1972 Oct;9(10):987–1011. doi: 10.1016/0019-2791(72)90110-3. [DOI] [PubMed] [Google Scholar]
- Werblin T. P., Siskind G. W. Effect of tolerance and immunity on antibody affinity. Transplant Rev. 1972;8:104–136. doi: 10.1111/j.1600-065x.1972.tb01566.x. [DOI] [PubMed] [Google Scholar]


