Abstract
Macrohage function, as measured by carbon clearance, differs in different inbred strains. Low affinity (KR) antibody response to HST is associated either with poor carbon clearance, or slow recovery from carbon blockade by macrophages.
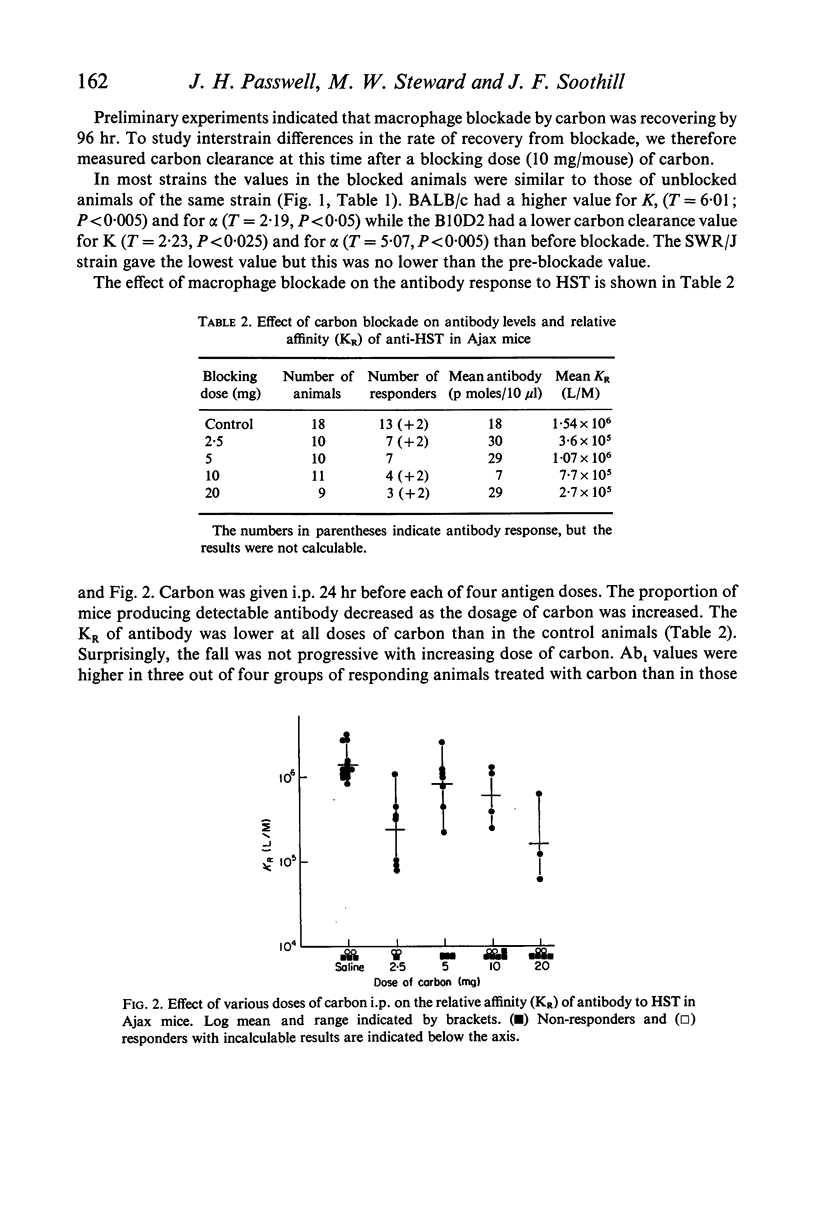
Carbon blockade reduces KR, but not Abt in mice with high carbon clearance and KR.
Stilboestrol increases carbon clearance and KR in mice with slow clearance and low KR.
The significance of these findings in pathogenesis and treatment of chronic soluble complex disease is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers J. H., Steward M. W., Soothill J. F. Differences in immune elimination in inbred mice. The role of low affinity antibody. Clin Exp Immunol. 1972 Sep;12(1):121–132. [PMC free article] [PubMed] [Google Scholar]
- BIOZZI G., BENACERRAF B., HALPERN B. N. Quantitative study of the granulopectic activity of the reticulo-endothelial system. II. A study of the kinetics of the R. E. S. in relation to the dose of carbon injected; relationship between the weight of the organs and their activity. Br J Exp Pathol. 1953 Aug;34(4):441–457. [PMC free article] [PubMed] [Google Scholar]
- DIXON F. J., FELDMAN J. D., VAZQUEZ J. J. Experimental glomerulonephritis. The pathogenesis of a laboratory model resembling the spectrum of human glomerulonephritis. J Exp Med. 1961 May 1;113:899–920. doi: 10.1084/jem.113.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M. Cell interactions in the immune response in vitro. II. The requirement for macrophages in lymphoid cell collaboration. J Exp Med. 1972 May 1;135(5):1049–1058. doi: 10.1084/jem.135.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLES H. M., HENDRICKSE R. G. Nephrosis in Nigerian children. Role of Plasmodium malariae, and effect of antimalarial treatment. Br Med J. 1963 Jul 6;2(5348):27–31. doi: 10.1136/bmj.2.5348.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchley C. J. The actvity of mouse Kupffer cells following intravenous injection of T4 bacteriophage. Clin Exp Immunol. 1969 Jul;5(1):173–187. [PMC free article] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison N. A. The immunogenic capacity of antigen taken up by peritoneal exudate cells. Immunology. 1969 Jan;16(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- OLD L. J., BENACERRAF B., CLARKE D. A., CARSWELL E. A., STOCKERT E. The role of the reticuloendothelial system in the host reaction to neoplasia. Cancer Res. 1961 Oct;21:1281–1300. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty R. E., Steward M. W., Soothill J. F. The heterogeneity of antibody affinity in inbred mice and its possible immunopathologic significance. Clin Exp Immunol. 1972 Oct;12(2):231–241. [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Haberkern R., Christian C. L. Experimental chronic glomerulitis. J Exp Med. 1968 Apr 1;127(4):819–832. doi: 10.1084/jem.127.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet T., Newlin C., Friedman H. Effects of RES "blockade" on antibody-formation. I. Suppressed cellular and humoral haemolysin responses in mice injected with carbon particles. Immunology. 1969 Apr;16(4):433–446. [PMC free article] [PubMed] [Google Scholar]
- Soothill J. F., Steward M. W. The immunopathological significance of the heterogeneity of antibody affinity. Clin Exp Immunol. 1971 Aug;9(2):193–199. [PMC free article] [PubMed] [Google Scholar]
- Souhami R. L. The effect of colloidal carbon on the organ distribution of sheep red cells and the immune response. Immunology. 1972 Apr;22(4):685–694. [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Allison A. C. Mode of action of adjuvants: effects on antibody responses to macrophage-associated bovine serum albumin. J Immunol. 1970 Jan;104(1):128–139. [PubMed] [Google Scholar]
- Steward M. W., Petty R. E. The antigen-binding characteristics of antibody pools of different relative affinity. Immunology. 1972 Dec;23(6):881–887. [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Petty R. E. The use of ammonium sulphate globulin precipitation for determination of affinity of anti-protein antibodies in mouse serum. Immunology. 1972 May;22(5):747–756. [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Voller A. The effect of malaria on the relative affinity of mouse antiprotein antibody. Br J Exp Pathol. 1973 Apr;54(2):198–202. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Askonas B. A., Allison A. C. A role of macrophages in the stimulation of immune responses by adjuvants. J Immunol. 1969 Jul;103(1):71–78. [PubMed] [Google Scholar]
- Unanue E. R., Askonas B. A. The immune response of mice to antigen in macrophages. Immunology. 1968 Aug;15(2):287–296. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Cerottini J. C. The function of macrophages in the immune response. Semin Hematol. 1970 Apr;7(2):225–248. [PubMed] [Google Scholar]


