Abstract
Objective:
To report abnormal changes in lower leg anterior compartment pressure in 1 subject who consumed creatine as a dietary supplement.
Background:
The subject received creatine at a dosage of 0.3 g·kg body mass−1·d−1 for 6 days, followed by 0.03 g·kg body mass−1·d−1 for 28 days. Thereafter, the subject consumed no supplement for 21 days. Compartment pressure was measured pre-exercise and for 15 minutes postexercise after a treadmill run for 20 minutes at 80% of VO2 max before supplementation and after 6 and 34 days of supplementation.
Differential Diagnosis:
Normally, resting anterior compartment pressure is less than 15 mm Hg, whereas postexercise pressure is below 30 to 35 mm Hg. Creatine supplementation for 6 days dramatically increased pressure at rest (31 mm Hg) and at 1 minute (67 mm Hg), 5 minutes (35 mm Hg), 10 minutes (28 mm Hg), and 15 minutes (26 mm Hg) postexercise. Pressure remained high at rest (35 mm Hg) and at 1 minute (109 mm Hg), 5 minutes (90 mm Hg), 10 minutes (87 mm Hg), and 15 minutes (69 mm Hg) postexercise after 34 days of supplementation.
Treatment:
The subject stopped taking creatine for 21 days. Compartment pressure was measured at rest and after exercise after 7 and 21 days with no supplementation. Anterior compartment pressure decreased after cessation of creatine supplementation. However, pressures were elevated at 7 days postsupplementation at rest (26 mm Hg) and at 1 minute (112 mm Hg), 5 minutes (58 mm Hg), 10 minutes (40 mm Hg), and 15 minutes (30 mm Hg) postexercise. Pressures at 21 days postsupplementation remained high at rest (24 mm Hg) and at 1 minute (64 mm Hg), 5 minutes (42 mm Hg), 10 minutes (27 mm Hg), and 15 minutes (27 mm Hg) postexercise.
Conclusion:
These data indicate that creatine supplementation can substantially increase anterior compartment pressure in the leg.
Keywords: ergogenic aids, phosphocreatine, sports medicine
Anterior compartment pressures of the lower leg were measured in 1 healthy, physically active, male subject (age, 25 years) during and after creatine supplementation. The subject was not a vegetarian dieter, was not currently using any prescription medication, had never used anabolic steroids, and had a normal resting blood pressure of 139/89 mm Hg or lower; he had not used creatine supplementation within 3 months of starting the investigation. The subject had no previous history of lower leg conditions involving the musculoskeletal, neurologic, or vascular structures. The subject read and signed an informed consent form and completed a health history questionnaire in accordance with guidelines set forth by the Advisory Committee for Human Experimentation at the University of Kansas.
DIAGNOSIS
Anterior compartment pressures were measured in the subject on 5 occasions. For each experimental session, he reported to the laboratory in a 3-hour postabsorptive state, having refrained from exercise for 48 hours and from caffeine, alcohol, and other potential diuretics for 24 hours before testing. During each experimental session, the subject's body mass was measured, and a muscle biopsy was performed (except for 21 days postsupplementation, when no muscle biopsy was performed); the subject then ran on a level treadmill at 80% of maximal aerobic power for 20 minutes. Anterior compartment pressure was measured at rest and after the run.
Resting and postexercise compartment pressures were measured with the subject in a supine position. A towel supported the knee, with the ankle in a neutral position and the first toe of the foot pointed vertically.1 Anterior compartment pressure was measured using a Stryker Intracompartment Pressure Monitor System (Stryker Instruments, Kalamazoo, MI). Resting pressures were taken when the musculature of the anterior compartment felt relaxed upon palpation. The point of catheter insertion into the anterior compartment was 15 cm distal to the tibial tuberosity and 2 cm lateral to the tibial crest.2 A 1-cm2 area of skin was sterilized with Betadine solution (Purdue Frederick Co, Norwalk, CT) and anesthetized with 1 mL of 1% lidocaine without epinephrine. The fascia was pierced at a 30° angle to the long axis of the leg, and the catheter was directed cephalad at a 45° angle into the muscle belly of the tibialis anterior and positioned 2 to 3 cm within the anterior compartment. The pressure monitor was filled with a saline solution containing heparin (Celsus, Inc, Cincinnati, OH) that provided a continuous fluid column from the catheter tip to the transducer. After catheter insertion, the pressure was allowed to stabilize (approximately 30 seconds) and was then recorded from the digital monitor display. After resting pressure was measured, the catheter was removed, and the subject began exercising. After a 5-minute warm-up at 50% of maximal aerobic power, the exercise intensity was increased to 80% of maximal aerobic power, and the subject ran for 20 minutes. Immediately after exercise, the subject was positioned as previously described, and the catheter was reinserted. Pressures were recorded immediately postexercise and every minute for 15 minutes. The data are reported for the pre-exercise period and for 1, 5, 10, and 15 minutes postexercise.
Muscle biopsies of the vastus lateralis were performed using procedures outlined by Bergström,3 with suction applied to the biopsy needle to aid in tissue extraction. The samples were immediately frozen in liquid nitrogen and stored at −70°C until analysis. The freeze-dried sample was powdered and analyzed for adenosine triphosphate, phosphocreatine, and creatine concentrations.4 Total creatine concentration was calculated as the sum of the phosphocreatine and free creatine concentrations.
TREATMENT
The subject received creatine monohydrate (NutraSense, Shawnee Mission, KS) in tablet form (1 g creatine monohydrate and 1.4 g dextrose). He was given creatine for 6 days in a loading phase (86.8 kg × 0.3 g·kg body mass−1·d−1), followed by a 28-day maintenance phase (88.5 kg × 0.03 g·kg body mass−1·d−1). The subject consumed the supplement in 4 equal doses throughout the day, according to the manufacturer's directions. He continued training during supplementation. After 34 days of supplementation, the subject was withdrawn from supplementation for 21 days. Muscle biopsies were collected, and anterior compartment pressure was measured at rest and after exercise before supplementation, after completion of 6 and 34 days of supplementation, and after 7 and 21 days without dietary creatine supplementation.
CLINICAL COURSE
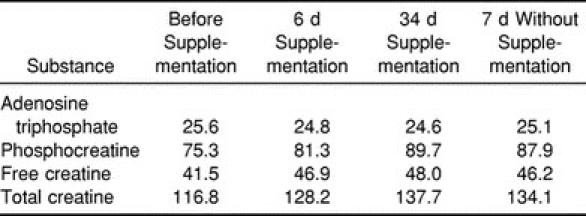
The subject's body mass increased from baseline by 1.7 kg at 6 days and remained elevated from baseline by 1.8 kg at 34 days of supplementation. Body mass decreased by 0.4 kg after 7 days without supplementation and by 0.7 kg after 21 days without supplementation. As expected, the dietary supplement protocol used in this study was successful in increasing the intramuscular levels of creatine, phosphocreatine, and total creatine relative to presupplementation levels (Table 1). Compared with presupplementation levels, phosphocreatine levels increased by 7.9% at 6 days and by 19.1% at 34 days. Creatine concentration increased by 13.0% at 6 days and 15.7% at 34 days, while total creatine increased by 9.8% at 6 days and 17.9% at 34 days. No significant changes in muscle adenosine triphosphate were observed at any measurement time. Phosphocreatine, creatine, and total creatine levels decreased slightly after 7 days without supplementation. The subject did not provide a muscle biopsy sample at the 21-day measurement time.
Table 1.
Vastus Lateralis Adenosine Triphosphate, Phosphocreatine, Free Creatine, and Total Creatine Concentrations (mmol·kg·d·m−1) Before, During, and After Creatine Supplementation
Compared with presupplementation measurements, creatine supplementation increased compartment pressure at rest, with markedly steeper increases in response to exercise (Table 2). Large increases in anterior compartment pressure were observed after both 6 and 34 days of dietary supplementation. Compared with presupplementation measurements, increases were observed at rest and at 1, 5, 10, and 15 minutes postexercise. For example, compared with presupplementation pressure, the resting compartment pressure was increased to 31 mm Hg after 6 days and 35 mm Hg after 34 days of supplementation. After 6 days of supplementation, the subject's 1-minute postexercise pressure was 67 mm Hg, and after 34 days, the subject's pressure was 109 mm Hg. The pressures at 5, 10, and 15 minutes postexercise at 6 and 34 days of supplementation were markedly elevated compared with presupplementation pressures.
Table 2.
Anterior Compartment Pressure (mm Hg) Before, During, and After Creatine Supplementation
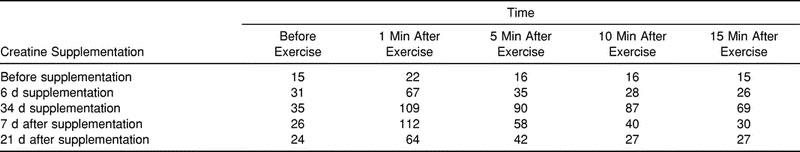
After 7 days without supplementation, resting anterior compartment pressure decreased to 26 mm Hg from the 35 mm Hg recorded at 34 days (Table 2). At 1 minute postexercise, the pressure was still elevated at 112 mm Hg. For the remainder of the postexercise measurement period, anterior compartment pressures were lower than those measured after 34 days of supplementation but still much higher than presupplementation pressures.
Anterior compartment pressure continued to decrease after 21 days without supplementation (Table 2). For example, resting pressure decreased to 24 mm Hg, while 1-minute postexercise pressure decreased to 64 mm Hg. Similar responses were observed in the 5-, 10-, and 15-minute pressure measurements.
DISCUSSION
Creatine dietary supplementation has been promoted as a beneficial ergogenic aid for athletes participating in certain athletic events. Creatine supplementation is thought to improve athletic performance by increasing intramuscular concentrations of creatine and phosphocreatine. The subject examined in our study had greater muscle phosphocreatine, creatine, and total creatine content after creatine supplementation compared with presupplementation content (Table 1). These data are similar to those reported by Harris et al,5 who observed a 20% increase in phosphocreatine in subjects supplemented with 5 g of creatine 4 to 6 times per day for 1 week. Similarly, Hultman et al6 demonstrated that taking 20 g of creatine per day for 6 days acutely elevated the total creatine content of muscle, and ingestion of 2 g of creatine per day for 30 days maintained the elevated total creatine concentration.
A decrease in fluid excretion has been observed during the early phases of creatine supplementation.6 A sodium-dependent process that actively transports creatine across the cell membrane has been documented and may be a factor in the observed changes in fluid homeostasis.7,8 This mechanism allows for the uptake of 1 creatine molecule with 2 sodium molecules. Several researchers9–11 have reported increases in body mass of 0.9 to 3.8 kg during the early phases of creatine supplementation and have suggested that the transport process is partly responsible for the increase in body mass.
Creatine uptake may contribute to the increased water transferred into the muscle fiber during supplementation, and as a result, stimulate a swelling of the muscle fiber.11 Additionally, the synthesis of new protein throughout the body after the later phase of supplementation may contribute to an increase in muscle fiber size.12 Because of the rigidity of the anterior compartment of the lower leg, an increase in water content or de novo protein synthesis in the muscle fiber will likely result in higher anterior compartment pressures at rest and after exercise.
Three factors should be considered when determining the presence of abnormally high tissue pressures in the anterior compartment of the lower leg. These factors include but are not limited to the geometric dimensions of the interstitial spaces, the physical characteristics of the surrounding tissues, and the total amount of fluid in the cell and the interstitial spaces.13 A normal increase of 20% in tissue volume of the lower leg compartment during exercise has been documented.14 This average increase in volume is due in part to increases in regional blood volume and transcapillary filtration of intravascular fluid.14–16 If the compartment is unable to accommodate this exercise-induced increase in fluid volume, pressure rises within the osteofascial space.17 Any change in muscle fiber size as a result of creatine supplementation might further limit the space available for normal increases in anterior compartment pressure. As a result of this space restriction, fluid within the elastic compartment may begin to shift from the vascular bed of the capillaries into the interstitial spaces. Capillary blood flow may be limited by the increased pressures surrounding the vessel, thus leading to ischemia. This effect would be followed by a resultant decrease in microcirculation,18 limiting function of the arterial and venous systems and further compromising nutrient blood flow, tissue oxygenation, and neuromuscular function and eventually leading to muscle necrosis.19
Measuring compartment pressures after the specific sport activity responsible for the increased pressure is the optimal method for identifying exercise-induced compartment syndrome. Pedowitz et al2 published criteria based on intramuscular anterior compartment pressures recorded with a slit catheter before and after exercise in 210 muscle compartments without chronic compartment syndrome. They recommended the following diagnostic criteria for abnormally elevated compartmental pressures: (1) a pre-exercise pressure of 15 mm Hg or greater; (2) a 1-minute postexercise pressure of 30 mm Hg or greater; or (3) a 5-minute postexercise pressure of 20 mm Hg or greater.2 After creatine supplementation, not only were the pressures that we observed higher at rest, but the pressures were substantially increased after exercise. The pressure responses were extremely elevated during the postexercise period after 6 and 34 days of supplementation. The resting and postexercise pressures were abnormally elevated after 6 days of supplementation and remained so after 34 days of supplementation. In each instance, the elevated pressures were observed in combination with a prolongation of the duration required for the pressures to return to pre-exercise levels. Clinically, the magnitude and duration of the compartment pressure are critical; if these abnormal pressures remain high, irreversible tissue damage results, particularly damage of the peroneal nerve. After 7 and 21 days without supplementation, the anterior compartment pressure was reduced compared with the pressure measured after 34 days of supplementation. It is attractive to speculate that the reduction in pressure resulted from a decrease in intramuscular creatine supplementation and a possible loss of muscle fiber size. A decrease in intramuscular phosphocreatine and creatine levels after the withdrawal of creatine supplementation has been reported.6 Intramuscular phosphocreatine and creatine levels decreased slightly after 7 days without supplementation. It is unfortunate that we were unable to obtain a 21-day, no-supplementation biopsy to evaluate the changes in phosphocreatine and creatine levels. This biopsy may have provided more insight into the relationship between changes in the intramuscular metabolites and anterior compartment pressure. Future studies should focus on the relationship between changes in anterior compartment pressure and creatine supplementation.
Individuals suffering from increased compartment pressures are expected to complain of lower extremity aching, cramping, or burning pain and sometimes tightness over the affected compartment.17 Symptoms resembling these complaints were noted by the subject after 6 and 34 days of supplementation. Subjective complaints of tightness and pain in the region of the anterior compartment during exercise were noted and continued for approximately 15 to 20 minutes after exercise ended. Consequently, despite the positive performance benefits that may result from creatine supplementation, the risk of abnormally elevating the pressures of the anterior compartment may contraindicate supplementation in some individuals.
It is important that medical personnel be aware of this side effect associated with creatine supplementation. Knowledge of this adverse effect is important in diagnosing those individuals more susceptible to lower leg injury and should enable the identification of potentially preventable adverse medical conditions. Stretching, cross-training, slow exercise progression, and avoiding ballistic movements may minimize the exacerbation of present symptoms, and modification of training programs should decrease the possible occurrence of debilitating conditions. Additional study of the effects of creatine supplementation on muscle blood flow, muscle function, and nerve conduction in the anterior compartment of the lower leg should be performed.
ACKNOWLEDGMENTS
This research was supported by the National Athletic Trainers' Association grant 399-A004 (Dallas, TX), Stryker Instruments (Kalamazoo, MI), and The NutraSense Co (Shawnee Mission, KS).
REFERENCES
- 1.Gershuni DH, Yaru NC, Hargens AR, Lieber RL, O'Hara RC, Akeson WH. Ankle and knee position as a factor modifying intracompartmental pressure in the human leg. J Bone Joint Surg Am. 1984;66:1415–1420. [PubMed] [Google Scholar]
- 2.Pedowitz RA, Hargens AR, Mubarak SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18:35–40. doi: 10.1177/036354659001800106. [DOI] [PubMed] [Google Scholar]
- 3.Bergström J. Muscle electrolytes in man: determination by neutron activation analysis on needle biopsy specimens, a study on normal subjects, kidney patients and patients with chronic diarrhoea. Scand J Clin Lab Invest. 1962;14(suppl 68):1–110. [Google Scholar]
- 4.Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest: methods and variance of values. Scand J Clin Lab Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- 5.Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Colch) 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- 6.Hultman E, Soderlund K, Timmons J, Cederblad G, Greenhaff PL. Muscle creatine loading in men. J Appl Physiol. 1996;81:232–237. doi: 10.1152/jappl.1996.81.1.232. [DOI] [PubMed] [Google Scholar]
- 7.Guimbal C, Kilimann MW. A Na(+)-dependent creatine transporter in rabbit brain, muscle, heart, and kidney: cDNA cloning and functional expression. J Biol Chem. 1993;268:8418–8421. [PubMed] [Google Scholar]
- 8.Moller A, Hamprecht B. Creatine transport in cultured cells of rat and mouse brain. J Neurochem. 1989;52:544–550. doi: 10.1111/j.1471-4159.1989.tb09154.x. [DOI] [PubMed] [Google Scholar]
- 9.Volek JS, Kraemer WJ, Bush JA, et al. Creatine supplementation enhances muscular performance during high-intensity resistance exercise. J Am Diet Assoc. 1997;97:765–770. doi: 10.1016/S0002-8223(97)00189-2. [DOI] [PubMed] [Google Scholar]
- 10.Soderlund K, Balsom PD, Ekblom B. Creatine supplementation and high intensity exercise: influence on performance and muscle metabolism. Clin Sci. 1994;87:120–121. [Google Scholar]
- 11.Balsom PD, Ekblom B, Soderlund K, Sjodin B, Hultman E. Creatine supplementation and dynamic high-intensity intermittent exercise. Scand J Med Sci Sports. 1993;3:143–149. [Google Scholar]
- 12.Volek JS, Duncan ND, Mazzetti SA, et al. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sports Exerc. 1999;31:1147–1156. doi: 10.1097/00005768-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Reneman RS, Jageneau AHJ. The influence of weighted exercise on tissue (intramuscular) pressure in normal subjects and patients with intermittent claudication. Scand J Clin Lab Invest Suppl. 1973;128:37–42. [PubMed] [Google Scholar]
- 14.Jacobsson S, Kjellmer I. Accumulation of fluid in exercising skeletal muscle. Acta Physiol Scand. 1964;60:286–292. doi: 10.1111/j.1748-1716.1964.tb02890.x. [DOI] [PubMed] [Google Scholar]
- 15.Kjellmer I. An indirect method for estimating tissue pressure with special reference to tissue pressure in muscle during exercise. Acta Physiol Scand. 1964;62:31–40. doi: 10.1111/j.1748-1716.1964.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 16.Wallensten R, Eklund B. Intramuscular pressures and muscle metabolism after short-term and long-term exercise. Int J Sports Med. 1983;4:231–235. doi: 10.1055/s-2008-1026040. [DOI] [PubMed] [Google Scholar]
- 17.Varelas FL, Wessel J, Clement DB, Doyle DL, Wiley JP. Muscle function in chronic compartment syndrome of the leg. J Orthop Sports Phys Ther. 1993;18:586–589. doi: 10.2519/jospt.1993.18.5.586. [DOI] [PubMed] [Google Scholar]
- 18.Bradley EL., III The anterior tibial compartment syndrome. Surg Gynecol Obstet. 1973;136:289–297. [PubMed] [Google Scholar]
- 19.Sheridan GW, Matsen FA, III, Krugmire RB., Jr Further investigations on the pathophysiology of the compartmental syndrome. Clin Orthop. 1977;123:266–270. [PubMed] [Google Scholar]


