Abstract
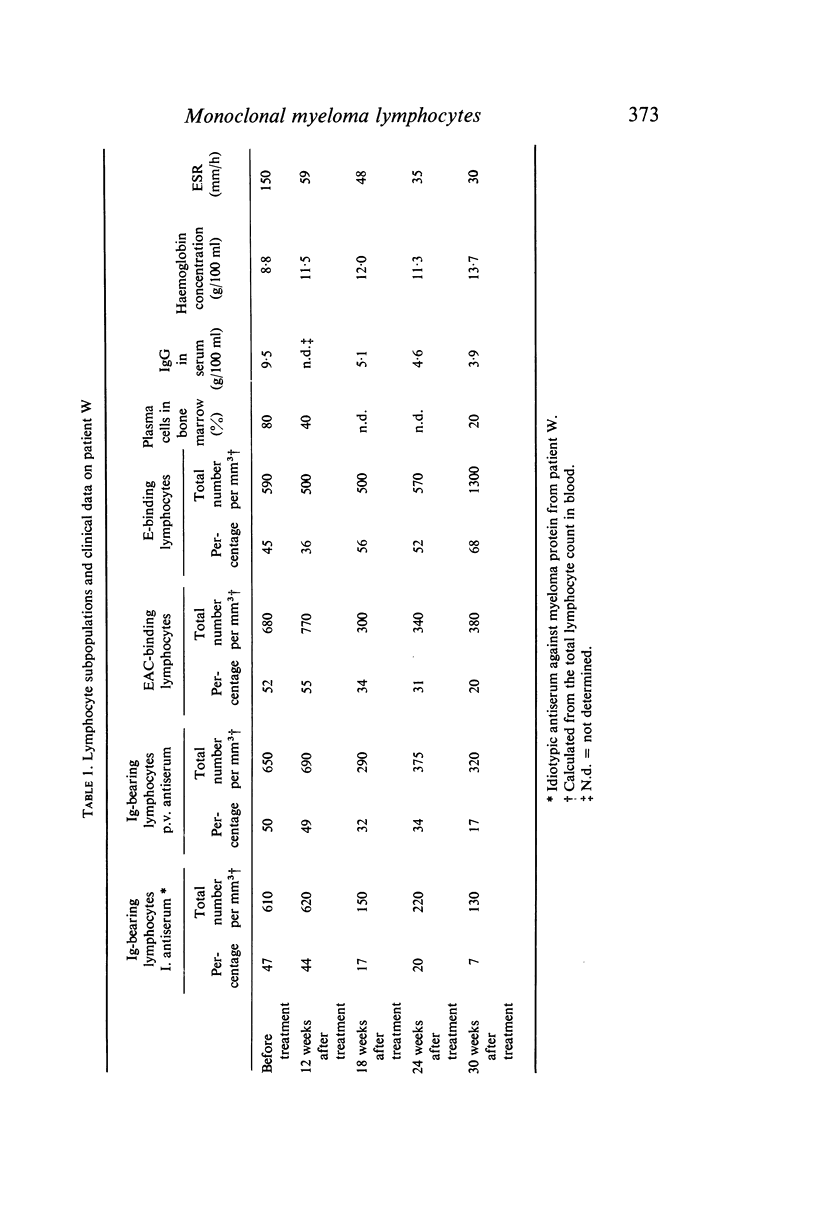
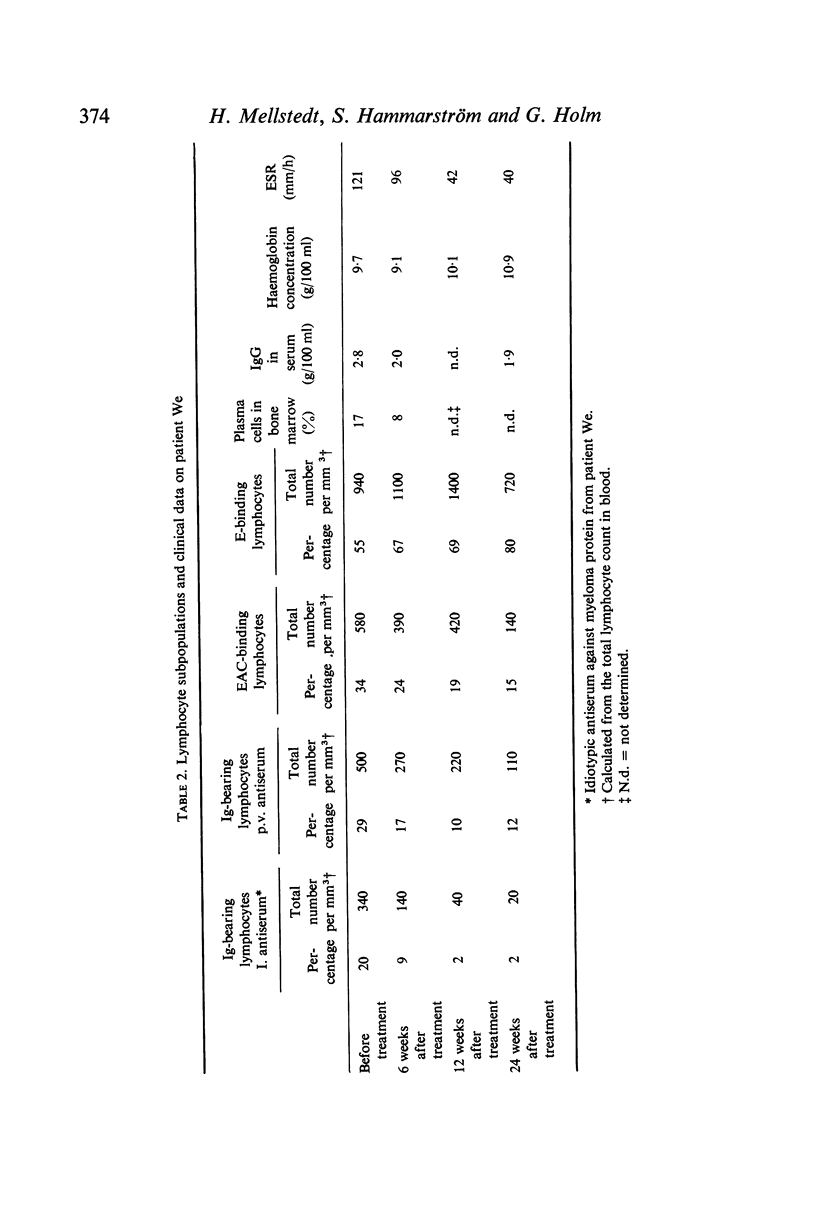
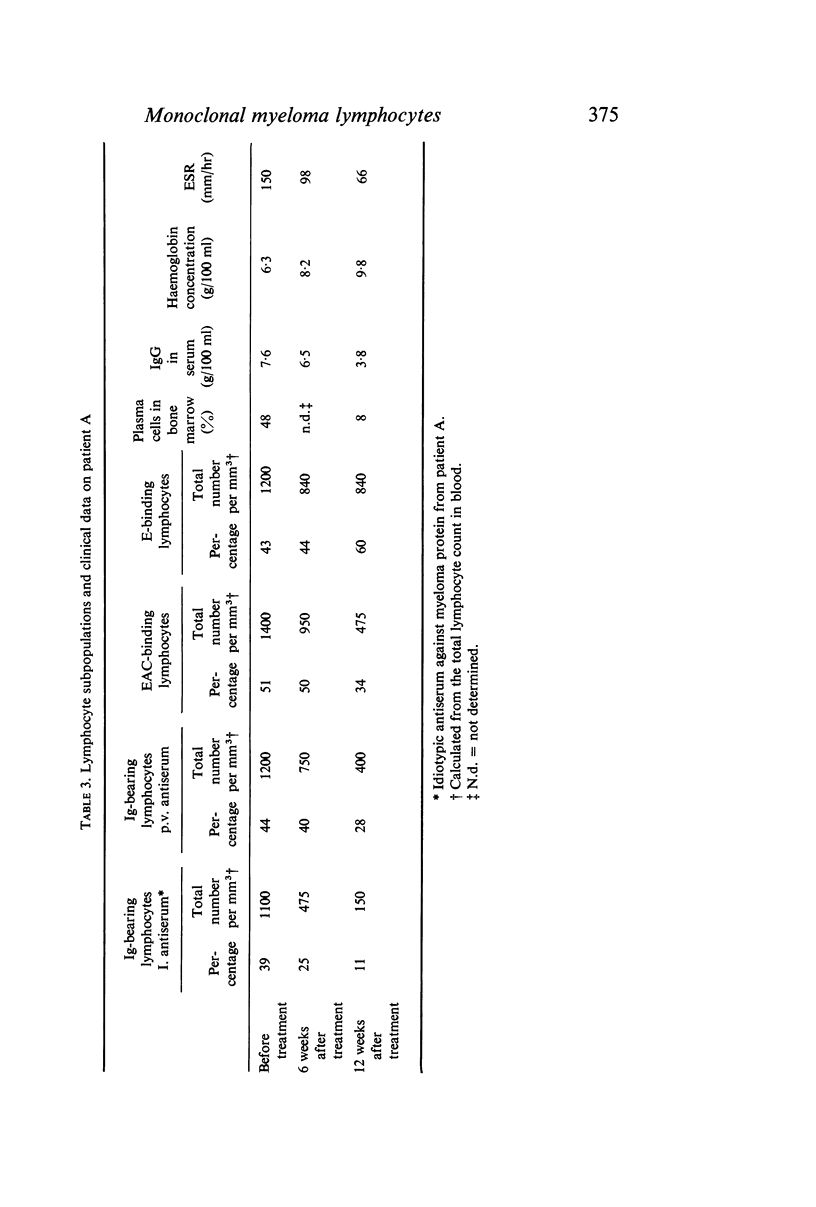
To identify monoclonal bone marrow-derived (B) lymphocytes in human myelomatosis specific rabbit antisera were produced against idiotypic specificities on IgG-κ myeloma proteins from three patients. The antisera neither cross-reacted nor reacted with normal immunoglobulins. By indirect immunofluorescence surface immunoglobulins were demonstrated on 20–47% of peripheral blood lymphocytes from untreated patients after staining with idiotypic antiserum against the patient's own myeloma protein, but not after staining with other idiotypic antisera. The antisera also stained autologous plasma cells. The monoclonal surface Ig on myeloma lymphocytes was removed by trypsin and regenerated after incubation in serum-free medium. Myeloma protein was not adsorbed onto lymphocytes. It is concluded that monoclonal B lymphocytes belonging to the plasma cell myeloma clone are present in myeloma patients. There were few normal B lymphocytes in untreated patients.
During treatment the monoclonal lymphocyte population and the plasma cells content in bone marrow, as well as the concentration of monoclonal immunoglobulin in serum, decreased simultaneously. These findings were associated with other signs of clinical improvement.
Full text
PDF



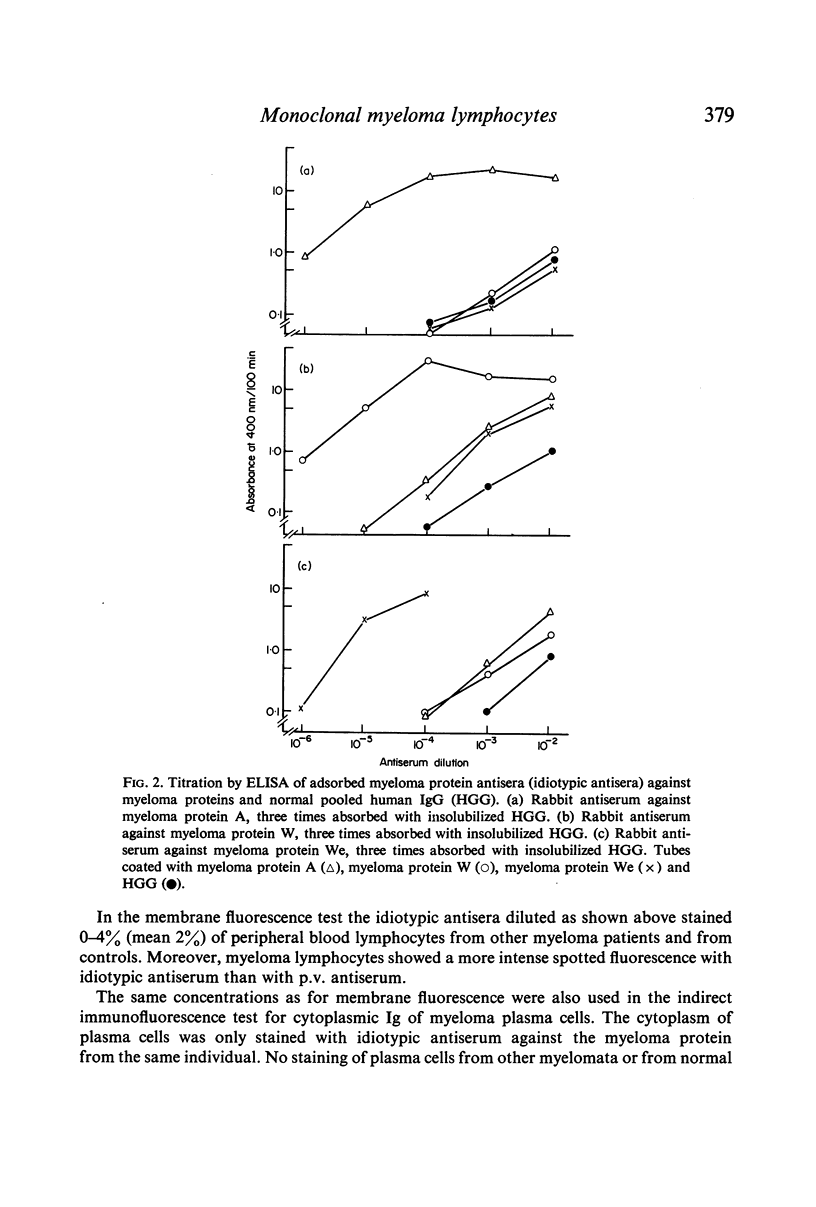
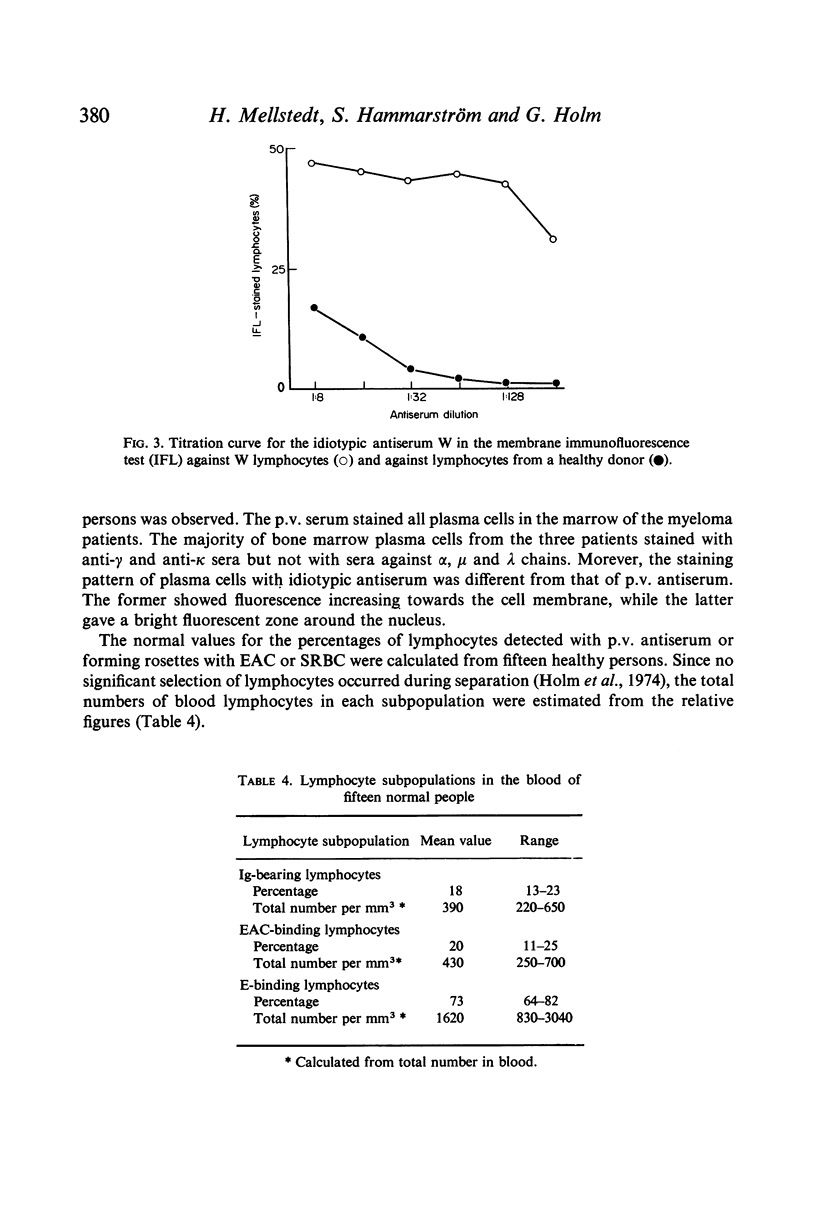




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton S. Growth-retarding mechanisms against lymphatic tumours. Search in mice and men. Transplant Rev. 1971;7:146–178. doi: 10.1111/j.1600-065x.1971.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- Cohn M., Notani G., Rice S. A. Characterization of the antibody to the C-carbohydrate produced by a transplantable mouse plasmacytoma. Immunochemistry. 1969 Jan;6(1):111–123. doi: 10.1016/0019-2791(69)90183-9. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Feizi T., Wernet P., Kunkel H. G., Douglas S. D. Lymphocytes forming red cell rosettes in the cold in patients with chronic cold agglutinin disease. Blood. 1973 Nov;42(5):753–762. [PubMed] [Google Scholar]
- Gutman A. B., Moore D. H., Gutman E. B., McClellan V., Kabat E. A. FRACTIONATION OF SERUM PROTEINS IN HYPERPROTEINEMIA, WITH SPECIAL REFERENCE TO MULTIPLE MYELOMA. J Clin Invest. 1941 Nov;20(6):765–783. doi: 10.1172/JCI101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller P., Yakulis V., Bhoopalam N., Costea N., Cabana V., Nathan R. D. Surface immunoglobulins on circulating lymphocytes in mouse and human plasmacytoma. Trans Assoc Am Physicians. 1972;85:192–202. [PubMed] [Google Scholar]
- Hopper J. E., Nisonoff A. Individual antigenic specificity of immunoglobulins. Adv Immunol. 1971;13:57–99. doi: 10.1016/s0065-2776(08)60183-2. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström F. D., Hardy W. R., Eberle B. J., Williams R. C., Jr Multiple myeloma and benign monoclonal gammopathy: differentiation by immunofluorescence of lymphocytes. Ann Intern Med. 1973 Jun;78(6):837–844. doi: 10.7326/0003-4819-78-6-837. [DOI] [PubMed] [Google Scholar]
- Lundgren G., Zukoski C. F., Möller G. Differential effects of human granulocytes and lymphocytes on human fibroblasts in vitro. Clin Exp Immunol. 1968 Oct;3(8):817–836. [PMC free article] [PubMed] [Google Scholar]
- Lynch R. G., Graff R. J., Sirisinha S., Simms E. S., Eisen H. N. Myeloma proteins as tumor-specific transplantation antigens. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1540–1544. doi: 10.1073/pnas.69.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellstedt H., Holm G. In vitro studies of lymphocytes from patients with plasma cell myeloma. I. Stimulation by mitogens and cytotoxic activities. Clin Exp Immunol. 1973 Nov;15(3):309–320. [PMC free article] [PubMed] [Google Scholar]
- Mellstedt H., Jondal M., Holm G. In vitro studies of lymphocytes from patients with plasma cell myeloma. II. Characterization by cell surface markers. Clin Exp Immunol. 1973 Nov;15(3):321–330. [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Paul W. E. Functional specificity of antigen-binding receptors of lymphocytes. Transplant Rev. 1970;5:130–166. doi: 10.1111/j.1600-065x.1970.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Potter M., Lieberman R. Genetics of immunoglobulins in the mouse. Adv Immunol. 1967;7:91–145. doi: 10.1016/s0065-2776(08)60127-3. [DOI] [PubMed] [Google Scholar]
- Potter M. The developmental history of the neoplastic plasma cell in mice: a brief review of recent developments. Semin Hematol. 1973 Jan;10(1):19–32. [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Immunoglobulins on the surface of lymphoid cells in Waldenström's macroglobulinemia. J Clin Invest. 1972 Mar;51(3):701–705. doi: 10.1172/JCI106858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet P., Feizi T., Kunkel H. G. Idiotypic determinants of immunoglobulin M detected on the surface of human lymphocytes by cytotoxicity assays. J Exp Med. 1972 Sep 1;136(3):650–655. doi: 10.1084/jem.136.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]


