Abstract
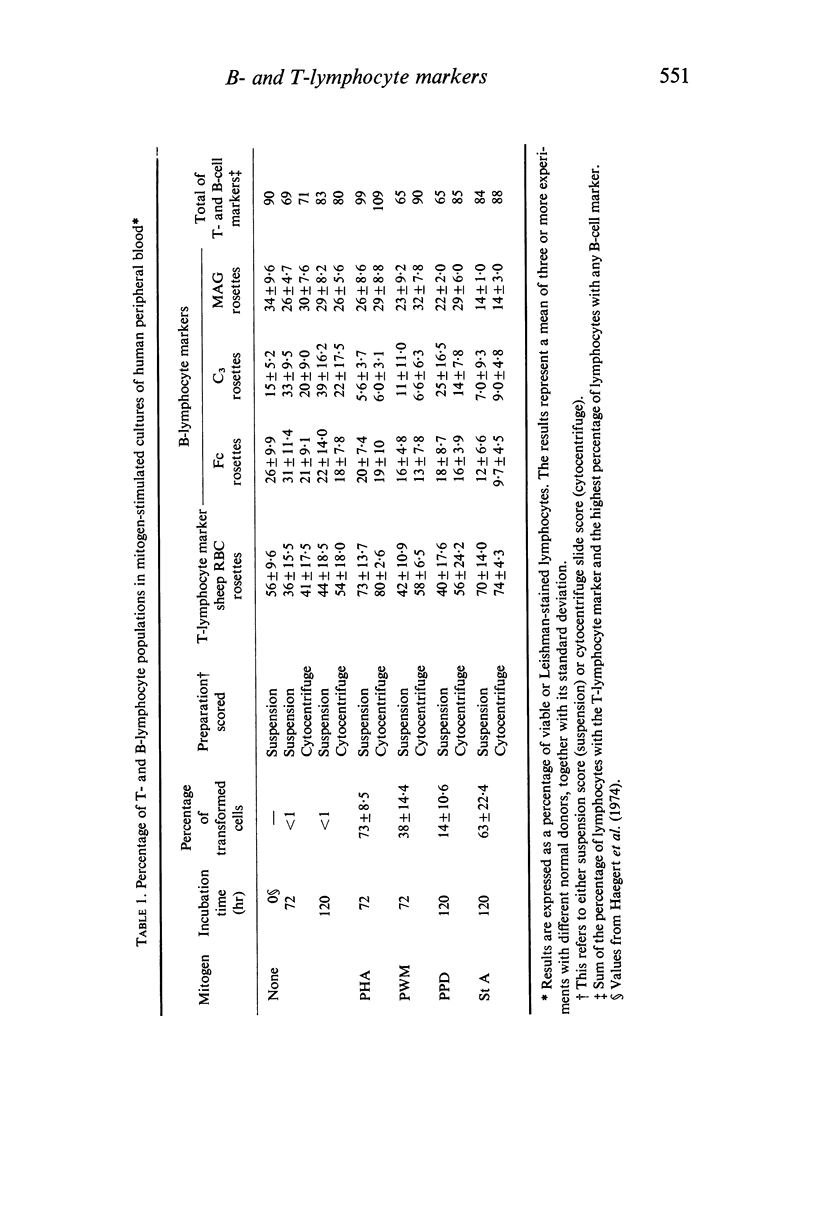
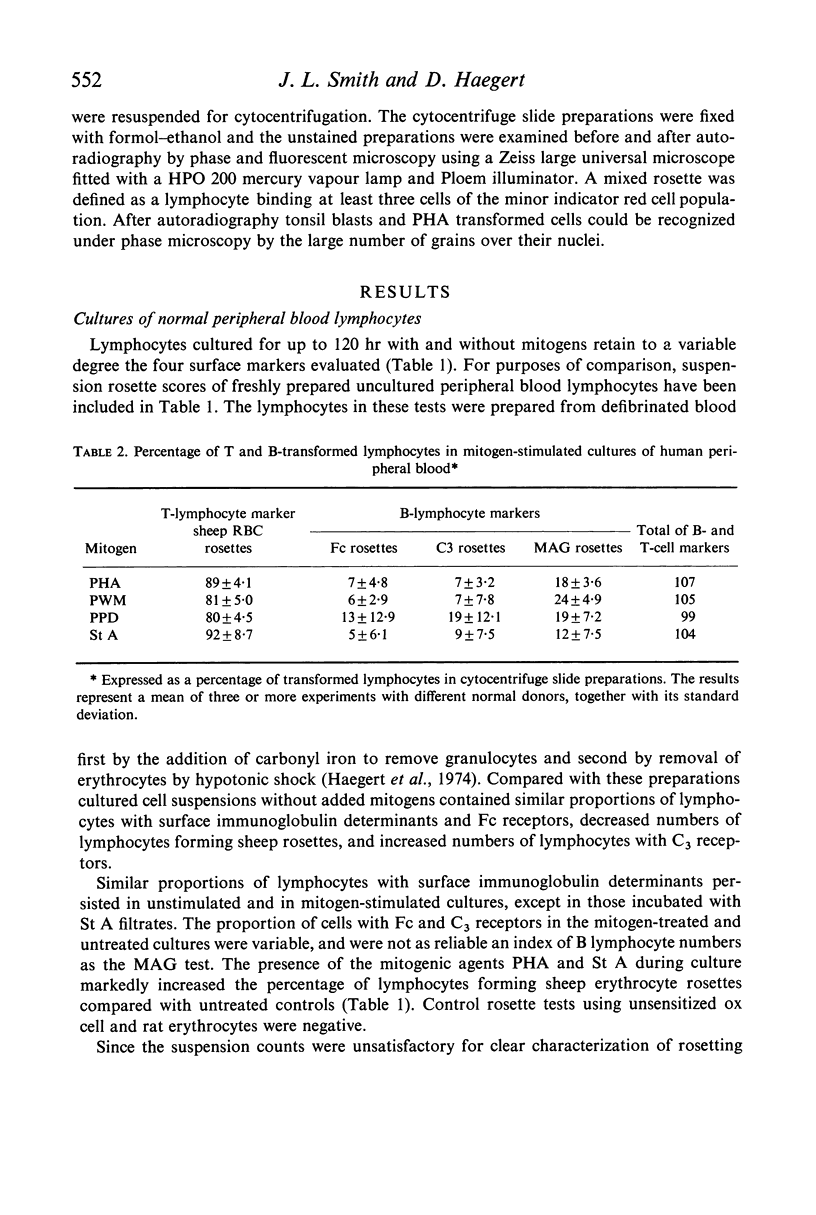
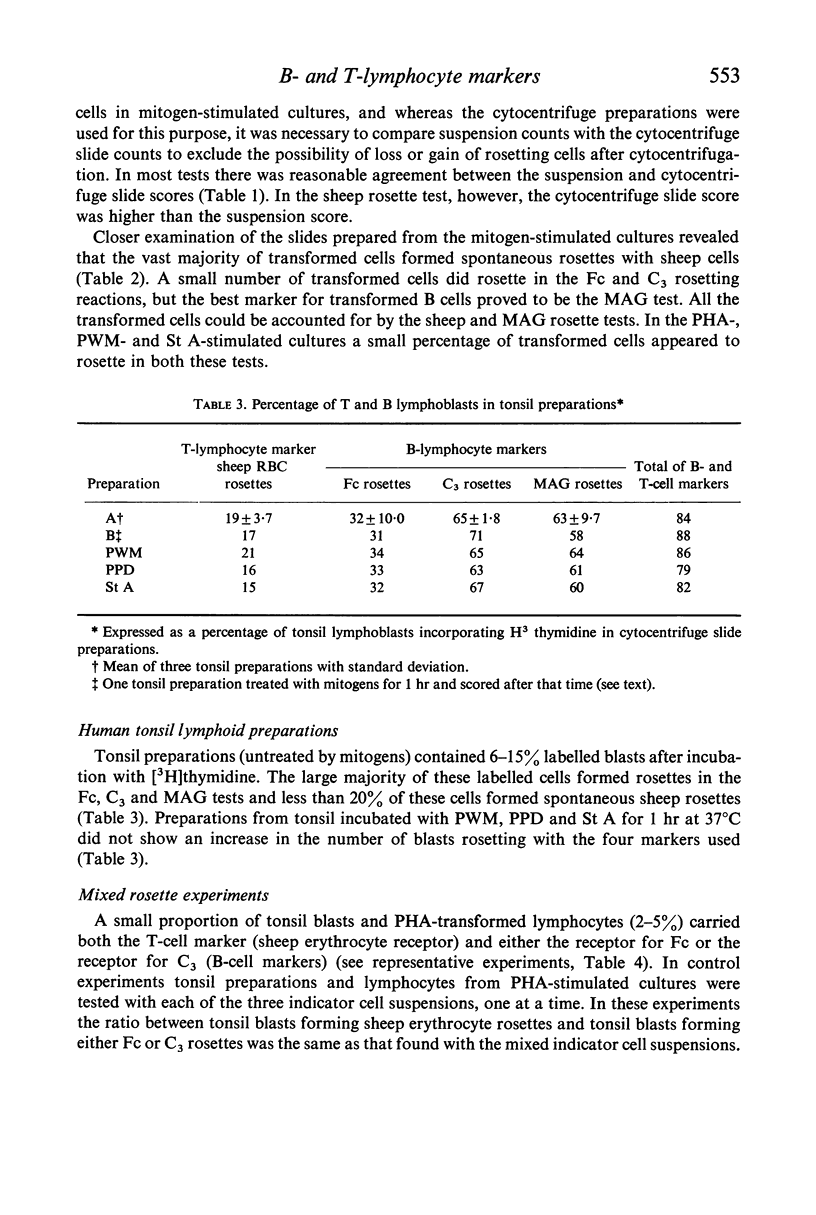
Mitogen-transformed human peripheral blood lymphocytes and tonsil blasts were examined by rosette formation to detect the presence of membrane-bound immunoglobulin (Ig) and surface receptors for fixed IgG and fixed C3. In addition, the capacity of these cells to rosette with sheep erythrocytes was evaluated as a reaction characteristic of T lymphocytes. In order for clear morphological recognition of the rosetting transformed lymphocytes and the rosetting tonsil blasts a cytocentrifuge technique was developed and used in conjunction with autoradiography and/or with Romanowsky stains. Using these techniques and the culture methods described in this paper phytohaemagglutinin, pokeweed mitogen, streptococcal filtrates and purified protein derivative stimulated predominantly T cells in the peripheral blood of man. A minority of the transformed cells in these mitogen-stimulated cultures (<24%) did rosette with B lymphocyte markers and presumably represent a B-cell response. No significant differences were found between the T- or B-cell specificity of the mitogens investigated. Lymphoid preparations from tonsils excised from normal donors with recurrent tonsillitis were found to contain 6–15% lymphoblasts and the large majority of these cells formed rosettes with the B-cell markers, less than 20% of these lymphoblasts formed spontaneous sheep erythrocyte rosettes. Using a mixed rosetting technique a small proportion (<5%) of PHA-transformed cells and tonsil lymphoblasts were found to have combined sheep Fc or combined sheep C3 receptors. The investigation of B- and T-lymphocyte surface markers on mitogentransformed lymphocytes was extended to neoplastic lymphocyte populations and it was found that the majority of transformed cells (> 70%) present in chronic lymphocytic leukaemia cultures stimulated with PHA after 6 days incubation were transformed T lymphocytes.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBROSE C. T. THE REQUIREMENT FOR HYDROCORTISONE IN ANTIBODY-FORMING TISSUE CULTIVATED IN SERUM-FREE MEDIUM. J Exp Med. 1964 Jan 1;119:1027–1049. doi: 10.1084/jem.119.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain P., Gordon J., Willetts W. A. Rosette formation by peripheral lymphocytes. Clin Exp Immunol. 1970 May;6(5):681–688. [PMC free article] [PubMed] [Google Scholar]
- Börjeson J., Reisfeld R., Chessin L. N., Welsh P. D., Douglas S. D. Studies on human peripheral blood lymphocytes in vitro. I. Biological and physicochemical properties of the pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):859–872. doi: 10.1084/jem.124.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R. D., Smith J. L., Clein G. P., Barker C. R. Absence of B- and T-cell markers on acute lymphoblastic leukaemic cells and persistence of the T-cell marker on mitogen-transformed T-lymphocytes. Br J Haematol. 1974 Apr;26(4):615–625. doi: 10.1111/j.1365-2141.1974.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Coombs R. R., Gurner B. W., Wilson A. B., Holm G., Lindgren B. Rosette-formation between human lymphocytes and sheep red cells not involving immunoglobulin receptors. Int Arch Allergy Appl Immunol. 1970;39(5-6):658–663. doi: 10.1159/000230390. [DOI] [PubMed] [Google Scholar]
- Dewhurst C. J., Harrison R. F., Harvey D. R., Parkinson C. E. Prediction of respiratory-distress syndrome. Lancet. 1973 Aug 11;2(7824):332–333. doi: 10.1016/s0140-6736(73)90847-7. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Adkinson N. F., Jr, Terry W. D. Evidence for individual human peripheral blood lymphocytes bearing both B and T cell markers. Nature. 1974 Jan 25;247(5438):213–215. doi: 10.1038/247213a0. [DOI] [PubMed] [Google Scholar]
- Douglas S. D. Electron microscopic and functional aspects of human lymphocyte response to mitogens. Transplant Rev. 1972;11:39–59. doi: 10.1111/j.1600-065x.1972.tb00045.x. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Cerottini J. C. Thymus-derived (T) cell immunoglobulins. Presence of a receptor site for IgG and absence of large amounts of "buried" Ig determinants on T cells. J Exp Med. 1972 Nov 1;136(5):1323–1328. doi: 10.1084/jem.136.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg T., Gurner B. W., Coombs R. R. Opsonic adherence of sensitized ox red cells to human lymphocytes as measured by rosette formation. Int Arch Allergy Appl Immunol. 1973;44(4):500–513. doi: 10.1159/000230956. [DOI] [PubMed] [Google Scholar]
- Harris A. W., Bankhurst A. D., Mason S., Warner N. L. Differentiated functions expressed by cultured mouse lymphoma cells. II. Theta antigen, surface immunoglobulin and a receptor for antibody on cells of a thymoma cell line. J Immunol. 1973 Feb;110(2):431–438. [PubMed] [Google Scholar]
- Havemann K., Rubin A. D. The delayed response of c0ronic lymphocytic leukemia lymphocytes to phytohemagglutinin in vitro. Proc Soc Exp Biol Med. 1968 Mar;127(3):668–671. doi: 10.3181/00379727-127-32769. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- LING N. R., HUSBAND E. M. SPECIFIC AND NON-SPECIFIC STIMULATION OF PERIPHERAL LYMPHOCYTES. Lancet. 1964 Feb 15;1(7329):363–365. doi: 10.1016/s0140-6736(64)92102-6. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Mendes N. F., Bianco C., Nussenzweig V. Binding of sheep red blood cells to a large population of human lymphocytes. Nature. 1971 Apr 23;230(5295):531–532. doi: 10.1038/230531a0. [DOI] [PubMed] [Google Scholar]
- Phillips B., Roitt I. M. Evidence for transformation of human B lymphocytes by PHA. Nat New Biol. 1973 Feb 21;241(112):254–256. doi: 10.1038/newbio241254a0. [DOI] [PubMed] [Google Scholar]
- Rubin A. D., Havemann K., Dameshek W. Studies in chronic lymphocytic leukemia: further studies of the proliferative abnormality of the blood lymphocyte. Blood. 1969 Feb;33(2):313–328. [PubMed] [Google Scholar]
- Smith J. L., Barker C. R. T and B cell separation by sheep-red-cell rosetting. Lancet. 1973 Mar 10;1(7802):558–559. doi: 10.1016/s0140-6736(73)90382-6. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Cawley J. C., Barker C. R. The response to plant mitogens of two subpopulations of lymphocytes in a case of chronic lymphocytic leukaemia. Clin Exp Immunol. 1973 Jul;14(3):397–408. [PMC free article] [PubMed] [Google Scholar]
- Smith J. L., Clein G. P., Barker C. R., Collins R. D. Characterisation of malignant mediastinal lymphoid neoplasm (Sternberg sarcoma) as thymic in origin. Lancet. 1973 Jan 13;1(7794):74–77. doi: 10.1016/s0140-6736(73)90469-8. [DOI] [PubMed] [Google Scholar]
- Wybran J., Chantler S., Fudenberg H. H. Isolation of normal T cells in chronic lymphatic leukaemia. Lancet. 1973 Jan 20;1(7795):126–129. doi: 10.1016/s0140-6736(73)90196-7. [DOI] [PubMed] [Google Scholar]
- Yoshida T. O., Andersson B. Evidence for a receptor recognizing antigen complexed immunoglobulin on the surface of activated mouse thymus lymphocytes. Scand J Immunol. 1972;1(4):401–408. doi: 10.1111/j.1365-3083.1972.tb03306.x. [DOI] [PubMed] [Google Scholar]


