Abstract
Objective:
To review the underlying pathophysiologic processes of concussive brain injury and relate these neurometabolic changes to clinical sports-related issues such as injury to the developing brain, overuse injury, and repeated concussion.
Data Sources:
Over 100 articles from both basic science and clinical medical literature selected for relevance to concussive brain injury, postinjury pathophysiology, and recovery of function.
Data Synthesis:
The primary elements of the pathophysiologic cascade following concussive brain injury include abrupt neuronal depolarization, release of excitatory neurotransmitters, ionic shifts, changes in glucose metabolism, altered cerebral blood flow, and impaired axonal function. These alterations can be correlated with periods of postconcussion vulnerability and with neurobehavioral abnormalities. While the time course of these changes is well understood in experimental animal models, it is only beginning to be characterized following human concussion.
Conclusions/Recommendations:
Following concussion, cerebral pathophysiology can be adversely affected for days in animals and weeks in humans. Significant changes in cerebral glucose metabolism can exist even in head-injured patients with normal Glasgow Coma Scores, underscoring the need for in-depth clinical assessment in an effort to uncover neurocognitive correlates of altered cerebral physiology. Improved guidelines for clinical management of concussion may be formulated as the functional significance and duration of these postinjury neurometabolic derangements are better delineated.
Keywords: metabolism, physiology, repeated concussion, traumatic brain injury
Concussion is defined as any transient neurologic dysfunction resulting from a biomechanical force. Loss of consciousness is a clinical hallmark of concussion but is not required to make the diagnosis. Other symptoms include confusion, disorientation, unsteadiness, dizziness, headache, and visual disturbances. These postconcussive deficits occur with minimal detectable anatomic pathology and often resolve completely over time, suggesting that they are based on temporary neuronal dysfunction rather than cell death. Neuronal dysfunction can occur due to ionic shifts, altered metabolism, impaired connectivity, or changes in neurotransmission. Thus, a complete understanding of the phenomenon of concussion requires knowledge of the underlying pathophysiology of this injury. In this article, we will review the neurometabolic events following experimental concussive brain injury and then apply this knowledge to specific scenarios pertinent to sport-related concussion.
POSTCONCUSSIVE PATHOPHYSIOLOGY
An Overview of Concussion Pathophysiology
Immediately after biomechanical injury to the brain, abrupt, indiscriminant release of neurotransmitters and unchecked ionic fluxes occur. The binding of excitatory transmitters, such as glutamate, to the N-methyl-D-aspartate (NMDA) receptor leads to further neuronal depolarization with efflux of potassium and influx of calcium. These ionic shifts lead to acute and subacute changes in cellular physiology.
Acutely, in an effort to restore the neuronal membrane potential, the sodium-potassium (Na+-K+) pump works overtime. The Na+-K+ pump requires increasing amounts of adenosine triphosphate (ATP), triggering a dramatic jump in glucose metabolism. This “hypermetabolism” occurs in the setting of diminished cerebral blood flow, and the disparity between glucose supply and demand triggers a cellular energy crisis. The resulting energy crisis is a likely mechanism for postconcussive vulnerability, making the brain less able to respond adequately to a second injury and potentially leading to longer-lasting deficits.
Following the initial period of accelerated glucose utilization, the concussed brain goes into a period of depressed metabolism. Persistent increases in calcium may impair mitochondrial oxidative metabolism and worsen the energy crisis. Unchecked calcium accumulation can also directly activate pathways leading to cell death. Intra-axonal calcium flux has been shown to disrupt neurofilaments and microtubules, impairing posttraumatic neural connectivity.
This overview represents a simplified framework of the neurometabolic cascade (Figure 1). Other important components of posttraumatic cerebral pathophysiology include, but are not limited to, generation of lactic acid, decreased intracellular magnesium, free radical production, inflammatory responses, and altered neurotransmission. We will now discuss some of the pertinent details of postconcussive pathophysiology in both experimental animal models and in humans.
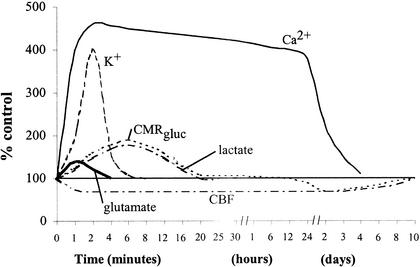
Figure 1.
Neurometabolic cascade following experimental concussion. K+, potassium; Ca2+, calcium; CMRgluc, oxidative glucose metabolism; CBF, cerebral blood flow. (Reprinted with permission. Giza CC, Hovda DA. Ionic and metabolic consequences of concussion. In: Cantu RC, Cantu RI. Neurologic Athletic and Spine Injuries. St Louis, MO: WB Saunders Co; 2000:80–100.).
Acute Metabolic and Ionic Changes
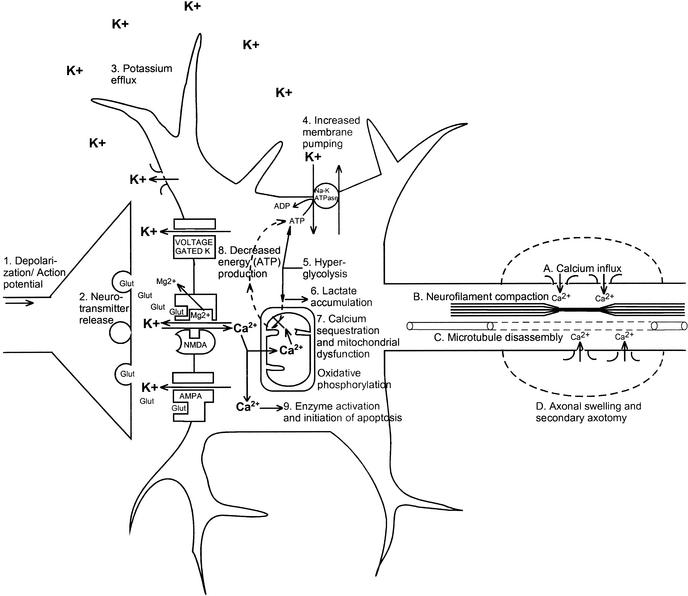
Immediately after biomechanical injury to the brain, there is disruption of neuronal membranes, axonal stretching, and opening of voltage-dependent K+ channels, which leads to a marked increase in extracellular K+.1–4 In addition, nonspecific depolarization leads to an early, indiscriminate release of the excitatory amino acid (EAA) glutamate, which exacerbates the K+ flux by activating kainate, NMDA, and D-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors (Figure 2, events 1 and 2). In rats, treatment with the EAA inhibitor kynurenic acid greatly diminishes the posttraumatic K+ efflux.3
Figure 2.
Neurometabolic cascade following traumatic injury. (1) Nonspecific depolarization and initiation of action potentials. (2) Release of excitatory neurotransmitters (EAAs). (3) Massive efflux of potassium. (4) Increased activity of membrane ionic pumps to restore homeostasis. (5) Hyperglycolysis to generate more adenosine triphosphate (ATP). (6) Lactate accumulation. (7) Calcium influx and sequestration in mitochondria leading to impaired oxidative metabolism. (8) Decreased energy (ATP) production. (9) Calpain activation and initiation of apoptosis. A, Axolemmal disruption and calcium influx. B, Neurofilament compaction via phosphorylation or sidearm cleavage. C, Microtubule disassembly and accumulation of axonally transported organelles. D, Axonal swelling and eventual axotomy. K+, potassium; Na+, sodium; Glut, glutamate; Mg2+, magnesium; Ca2+, calcium; NMDA, N-methyl-D-aspartate; AMPA, d-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid.
Normally, excessive extracellular K+ is taken up by surrounding glial cells.5–7 By this mechanism, the brain can maintain physiologic K+ levels after mild perturbations; however, larger insults, such as brain trauma or ischemia, overcome this compensation.8–11 As extracellular K+ increases, neuronal depolarization is triggered, leading to further release of EAAs, opening of EAA receptor channels (NMDA, AMPA, kainate), and still greater K+ flux (Figure 2, event 3). This massive excitation is then followed by a wave of relative neuronal suppression that has been termed spreading depression.12–16 One important distinction between classic spreading depression and postconcussive K+ fluxes is that after trauma, diffuse areas of the brain are affected simultaneously. Early loss of consciousness, amnesia, or other cognitive dysfunction may be manifestations of a posttraumatic spreading depression–like state.
In an effort to restore ionic homeostasis, energy-requiring membrane pumps are activated17–19 and trigger an increase in glucose use (Figure 2, events 4 and 5).20–22 This increase in glucose use occurs almost immediately after fluid percussion injury in rats and persists for up to 30 minutes in the ipsilateral cortex and hippocampus.22 After more severe injury such as cortical contusion, increased glucose metabolism may last 4 hours in areas distant from the contusion core.23 Because cerebral oxidative metabolism typically runs near its maximum, an abrupt increase in energy requirements is best met by an increase in glycolysis.24,25
Accelerated glycolysis leads to increased lactate production and is seen after both ischemic26–28 and concussive29–33 brain injury. In addition to hyperglycolysis, oxidative metabolism is also impaired after brain trauma.34–36 This impairment of mitochondrial function can lead to reduced ATP production, which provides a second stimulus for increased glycolysis. Thus, lactate production by glycolysis increases concurrent with a decrease in lactate metabolism, resulting in lactate accumulation (Figure 2, event 6). Elevated lactate levels can result in neuronal dysfunction by inducing acidosis, membrane damage, altered blood brain barrier permeability, and cerebral edema.37–41 Increased levels of lactate after traumatic brain injury (TBI) may leave neurons more vulnerable to a secondary ischemic injury,42 but whether this is the case in repeated traumatic injury is not known. An intriguing hypothesis suggests that glial lactate production increases posttraumatically and that this excess lactate is actually transported into neurons for use as an alternate fuel.43
Cerebral Blood Flow–Glucose Metabolism Uncoupling
Under normal conditions, cerebral blood flow (CBF) is tightly coupled to neuronal activity and cerebral glucose metabolism. After experimental fluid percussion injury, however, CBF may be reduced to 50% of normal.44–47 This posttraumatic decrease in CBF does not approach the 85% reduction seen in frank ischemia48; nonetheless, in a setting of increased glucose use (hyperglycolysis), this mismatch in supply and demand results in a potentially damaging energy crisis.
Calcium Influx, Mitochondrial Dysfunction, and Delayed Glucose Hypometabolism
Calcium accumulation is seen within hours of experimental concussion and may persist for 2 to 4 days.49–52 The posttraumatic depolarization and K+ efflux triggers the release of EAAs that, in turn, activates NMDA receptors.3 Activated NMDA receptors form a pore through which calcium (Ca2+) can enter the cell. A potent N-type calcium channel blocker, SNX-111, significantly reduces postconcussive Ca2+ accumulation,53 presumably by reducing release of glutamate.54 Results of treatment with NMDA receptor antagonists have been mixed, however. No reduction in Ca2+ accumulation was seen after weight-drop injury and pre-treatment with the NMDA receptor blocker MK-801,55 but treatment with HU-211, a synthetic cannabinoid with a pharmacologic profile characteristic of an NMDA receptor antagonist, was associated with a reduction in post-TBI Ca2+ accumulation.56
Excess intracellular Ca2+ may also be sequestered in mitochondria,34,36 resulting in impaired oxidative metabolism and, ultimately, energy failure (Figure 2, events 7 and 8). Cytochrome oxidase histochemistry, which is a measure of oxidative metabolism, shows a biphasic reduction after experimental concussion. In the ipsilateral cortex, a relative reduction on day 1 recovers by day 2, only to be reinstated on day 3, to bottom out on day 5, and to recover by 10 days postinjury. Smaller but more lasting changes are seen in the ipsilateral hippocampus, with decreases still evident at 10 days.57
After the initial period of hyperglycolysis, cerebral glucose use is diminished by 24 hours postinjury and remains low for 5 to 10 days in experimental animals.22 Positron emission tomography (PET) in humans shows similar decreases in global cerebral glucose metabolism that may last 2–4 weeks post-TBI.58 In addition, this study found that post-injury hypometabolism did not correlate closely with level of consciousness as measured by Glasgow Coma Score (GCS). Depressed cerebral glucose metabolism was seen in comatose patients as well as in walking, talking patients, suggesting that significant neurometabolic abnormalities may occur in the absence of overt clinical symptoms.58 What is not yet clear is whether this hypometabolism represents a period when the brain is relatively protected from secondary injury or if the brain is more vulnerable because it is unable to respond adequately to further energy demands. It is also unknown whether this depressed glucose metabolism is responsible for more subtle neurocognitive deficits seen after TBI.
Reductions in Magnesium
Intracellular magnesium levels are also immediately reduced after TBI and remain low for up to 4 days.59–62 This reduction in magnesium has been correlated with postinjury neurologic deficits, and pretreatment to restore magnesium levels results in improved motor performance in experimental animals.63 Decreased magnesium levels may lead to neuronal dysfunction via multiple mechanisms. Both glycolytic and oxidative generation of ATP are impaired when magnesium levels are low. Magnesium is necessary for maintaining the cellular membrane potential and initiating protein synthesis. Finally, low levels of magnesium may effectively unblock the NMDA receptor channel more easily, leading to greater influx of Ca2+ and its potentially deleterious intracellular consequences.
Diffuse Axonal Injury
Mechanical stretching of axons may result in membrane disruption and even depolarization.4 Increased axolemmal permeability persists for up to 6 hours postinjury64,65 and can lead to influx of Ca2+ (Figure 2, event 9A) and mitochondrial swelling.66,67 Neurofilament compaction occurs from 5 minutes to 6 hours postinjury (Figure 2, event 9B), either by phosphorylation, which alters neurofilament stability,68–70 or by calpain-mediated proteolysis of sidearms, which can lead to neurofilament collapse.71 Increased axonal Ca2+ levels have been shown to lead to microtubule breakdown from 6 to 24 hours after the initial injury (Figure 2, event 9C).72,73
Intra-axonal cytoskeletal abnormalities lead to accumulation of organelles at the site of axonal damage due to continued axonal transport along intact segments. These focal axonal swellings eventually develop constrictions that then lead to secondary axotomy (Figure 2, event 9D) and formation of axonal bulbs.73,74 Signs of secondary axonal disconnection may be seen as soon as 4 hours postinjury but have been reported to persist over days and even weeks in brain-injured humans.75
Delayed Cell Death and Persistent Calcium Accumulation
Post-TBI increases in Ca2+ do not inevitably lead to cell death. As mentioned previously, elevated intracellular Ca2+ may certainly lead to impaired mitochondrial metabolism, but neurons may still survive. In fact, after moderate experimental concussion, Ca2+ accumulation peaks in 2 days and resolves without obvious morphologic damage by 4 days.50 Animals experiencing more severe injury and demonstrating anatomic damage show persistent Ca2+ elevations at the injury site. In adult animals, a delayed rise (14 days postinjury) of Ca2+ is seen in distant structures (thalamus), which corresponds to neuronal death.52
Intracellular Ca2+ may trigger cell death by a variety of mechanisms (Figure 2, event 9), including overactivation of phospholipases,76 plasmalogenase, calpains,77,78 protein kinases,79 nitric oxide synthase, and endonucleases. These alterations may then lead to free radical overproduction,80 cytoskeletal reorganization,81 and activation of apoptotic genetic signals.82
Neurotransmitter Alterations
Long-term deficits in memory and cognition in a setting of minimal anatomic change are often seen after concussion. These may result from dysfunctional excitatory neurotransmission. Postconcussive alterations have been reported in glutamatergic (NMDA),83–85 adrenergic,86,87 and cholinergic88 systems. Long-term potentiation, an NMDA-dependent measure of plasticity, may be persistently impaired in the hippocampus after concussive brain injury.89–91 Concussive brain injury also leads to early changes in choline acetyltransferase activity88 and later loss of forebrain cholinergic neurons.92 Impaired cholinergic neurotransmission leads to learning and spatial memory deficits in animals.93,94
Inhibitory neurotransmission is also altered after TBI. A loss of γ-aminobutyric acid-producing (GABAergic) hilar neurons can compromise normal inhibition of hippocampal dentate granule cells.95 This loss of inhibitory neurons may predispose the traumatized brain to subsequent development of seizures.96
CONCERNS RELEVANT TO ATHLETIC CONCUSSION
Concussion in the Developing Brain
With increasing numbers of children and young adults participating in organized sports and sustaining head injuries, understanding the effects of TBI on the immature brain becomes more and more important. Clinical dogma has held that the younger the brain, the more resilient it is after injury. Recent studies of moderate fluid percussion in juvenile rats would seem to support this contention, demonstrating no obvious neurologic or pathologic deficits in these young animals.97,98 Using a closed head injury weight-drop model, significant deficits are seen only at injury severities that result in very high mortality (75%).99
On the other hand, there is also evidence to support the idea of specific developmental periods when the young brain may be more vulnerable to injury. TBI in children results in higher mortality than in adolescents, perhaps due to an increased incidence of cerebral edema.100–102 In the experimental model of developmental concussion, the youngest rats became hypotensive after even mild injuries and tended to have longer apnea times than adult rats. After severe fluid percussion injury, mortality in these immature animals approached 100%.97
It is reasonable to hypothesize that diffuse mechanical injury can have lasting effects on the complex sequence of neurochemical and anatomical events occurring during normal development. Indeed, long-term follow-up studies demonstrate persistent neurocognitive deficits after pediatric TBI.103,104 However, it is also difficult to assess developmental deficits in children after mild brain injury, as signs of overt neurologic dysfunction may be lacking, and loss of developmental potential may only be demonstrable at a later time or under specific circumstances.
Environmental enrichment provides a useful experimental model with which to study developmental plasticity after brain injury. In an enriched environment, rats are reared communally in a large cage with multiple toys, tunnels, and objects that are changed regularly. When compared with animals reared in standard conditions, enriched rats have increases in cortical thickness, larger neurons, more glia, a greater number of synapses, and enhanced dendritic branching.105–107 The enriched rats are also superior in cognitive testing using the Morris water maze.108 However, when moderately concussed juvenile rats are reared in an enriched environment, they fail to develop the increased cortical thickness and enhanced cognitive performance seen in sham-injured enriched controls.109 These results demonstrate that developmental brain injury, even without early behavioral deficits or significant later morphologic damage, can lead to impaired plasticity. Further studies must be done to determine whether this loss of experience-dependent plasticity is permanent or whether it represents a window of impairment after which the capacity for neural reorganization recovers.
Overuse Injury
As demonstrated by the recent focus on concussion in football and hockey, both athletic trainers and athletes feel significant pressure to return athletes to practice and play as soon as possible after injury. Although returning to play may be delayed because of concerns about susceptibility to a second brain injury, returning to practice might seem like a reasonable means of maintaining physical conditioning while awaiting full recovery.
In animals, the importance of limb use in recovery of function after unilateral cortical lesions has been well demonstrated.110 In fact, recovery of function was associated with increased dendritic growth in the homotopic region of the uninjured cortex, dependent on use of the intact forelimb. However, restraint of the uninjured forelimb, and thus forced overuse of the injured limb, resulted in a failure of dendritic enhancement in the intact cortex, an increase in the lesion size in the injured cortex, and a longer-lasting behavioral deficit.111 There also appears to be a time window when the deleterious effects of forced overuse are mitigated to some degree. In the same model, when immobilization of the intact arm was delayed 1 week after the injury, the functional recovery was still delayed, but the increase in lesion size did not occur.112 The results of these studies suggest that, at least after focal brain injury, it is possible to overstimulate the injured brain and that this excessive activation can lead to longer-lasting deficits.
Repeated Concussion
How soon to return to play after a head injury and the consequences of repeated concussions are two of the most important health-related issues in sports today. We have earlier reviewed what is known about the neurometabolic cascade of events that occurs after experimental brain injury (Figure 1). Acute abnormalities include ionic fluxes, indiscriminate glutamate release, hyperglycolysis, lactate accumulation, and axonal injury. Later steps in this physiologic cascade involve increased intracellular calcium, mitochondrial dysfunction, impaired oxidative metabolism, decreased glycolysis, diminished CBF, axonal disconnection, neurotransmitter disturbances, and delayed cell death. It is during this postinjury period, when cellular metabolism is already stretched to its limits, that the cell is more vulnerable to further insults.13 Examining the time course of the post-TBI neurometabolic cascade may help us determine guidelines for vulnerability of the concussed brain to a second injury.
Several physiologic parameters indicate windows of potential vulnerability in the traumatized brain. First, consider the period of glucose metabolism-CBF uncoupling. This phenomenon is most dramatic during the hyperglycolytic phase, which, in the rat, begins at the time of injury and lasts for at least 30 minutes.22 At this time, cerebral metabolism is already at its limit, and any further demand in energy (due to increased ionic flux) or reduction in energy (due to impaired blood flow or reduced ATP synthesis) may tip the scale in favor of irreversible neuronal injury. Thus, injured cells may be capable of recovering after an initial injury, but a second concussion during this energy crisis can lead to cell death. After the initial hyperglycolytic period, cerebral glucose metabolism is reduced, as is CBF, apparently resolving the mismatch in energy supply and demand. However, during this period, CBF may be unable to respond to a stimulus-induced increase in cerebral glucose metabolism, reinstating the metabolic crisis. An increase in glycolysis in this period may be due to excessive external stimulation or a second injury (concussion, ischemia, or seizure).
A second potential period of vulnerability centers on intracellular Ca2+ accumulation. Increased Ca2+ levels may impair mitochondrial metabolism at the time when the cell can least tolerate a reduction in ATP production. Additional Ca2+ influx, again due to increased physiologic stimulation or a second injury, may go on to activate proteases that initiate the march to programmed cell death. In the rat model, this period of acute Ca2+ accumulation is somewhat severity dependent and lasts 2 to 4 days.50,52
Another period of risk may be associated with impaired neurotransmission. Alterations in NMDA receptor composition can persist for up to 1 week after injury in developing rats,84 and a second injury in this period can lead to further impairment of excitatory neurotransmission with a greater degree of cognitive dysfunction. Long-term potentiation, postulated as a mechanism for learning and memory, is impaired for up to 8 weeks after experimental brain injury91 and may be another means by which altered excitatory neurotransmission results in neurobehavioral deficits. Diminished attention and cognition are particularly important in an athletic setting, when subtle impairments will likely increase the risk of recurrent head injury.
Post-TBI changes in inhibitory neurotransmission seen in rats95,96 can leave neurons more susceptible to massive depolarization and EAA release after a recurrent concussion. Excessive excitation may then more easily lead to seizure activity, increased energy demand, and possibly further cell death.
As each of these physiologic parameters has its own time frame, and each head injury can be very different from the next, it is difficult to definitively state the true duration of vulnerability to a second injury. Preliminary studies using a double concussion model in rats revealed increased anatomic damage and prolonged hypometabolism when 2 concussions were separated by as much as 5 hours.114 Double concussion also appears to increase immunostaining for glial fibrillary acidic protein (a marker for gliosis and scarring) and lead to greater cell loss when compared with a single injury.115 Interestingly, in a recent report, multiple mild concussions preceding a more severe concussion by 3 to 5 days actually resulted in a better functional outcome than an isolated severe concussion.116 However, the anatomic injury appeared unchanged. This finding raises the possibility that after TBI, there is a period of increased danger to a second injury, followed by a period when the brain may actually be more capable of recovering from a repeated injury.
Of course, translating these experimental time frames into time frames relevant to human concussion can be tricky. Posttraumatic derangements in glucose metabolism resolve within 7 to 10 days in rats, whereas in humans, persistent depression of glucose uptake has been reported 2 to 4 weeks later.58 Evidence of secondary axotomy is seen as soon as 4 hours postinjury in animals, but evidence of ongoing axonal damage has been reported in human brain tissue even weeks after trauma.75 In general, the time frame for events in rats is much shorter than for similar periods in humans, and it would not be unreasonable to assume that these periods of postinjury physiologic change are longer lasting in humans. In addition, differences in injury type and severity certainly affect the duration of these neurometabolic changes and must also be considered when determining back-to-play status.
SUMMARY
Cerebral concussion is followed by a complex cascade of ionic, metabolic, and physiologic events. The earliest changes are an indiscriminate release of EAAs and a massive efflux of K+, triggering a brief period of hyperglycolysis. This is followed by more persistent Ca2+ influx, mitochondrial dysfunction with decreased oxidative metabolism, diminished cerebral glucose metabolism, reduced CBF, and axonal injury. Late events in the cascade include recovery of glucose metabolism and CBF, delayed cell death, chronic alterations in neurotransmission, and axonal disconnection. Clinical signs and symptoms of impaired coordination, attention, memory, and cognition are manifestations of underlying neuronal dysfunction, most likely due to some of the processes described above. It is difficult to match clinical signs with specific underlying physiologic derangements, and guidelines permiting return to play only after resolution of all motor and cognitive deficits are a minimal precaution. There is recent evidence that even relatively asymptomatic (GCS 13-15) patients may demonstrate depressed glucose metabolism on PET imaging following TBI, reinforcing the need for meticulous clinical assessment. Further study will reveal more details of the time course for this neurometabolic cascade. This will eventually permit improved clinical monitoring of posttraumatic pathophysiology in actual patients, including variables such as cerebral glucose metabolism, blood flow, neuronal activity, and even molecular changes.
Traumatic injury to the developing brain may lead to long-lasting changes in cognitive potential, perhaps even with little evidence of an initial deficit. Children and adolescents who sustain a concussive brain injury should be closely monitored over time for the later appearance of neurobehavioral abnormalities.
Repeated injury within a particular time frame can lead to a much larger anatomical or behavioral impairment than 2 isolated injuries. The second injury may be obvious, such as a repeated concussion, hypoxia, or seizure, or it may occur in the form of premature activation or overstimulation of the injured brain. An awareness and understanding of postconcussive pathophysiology will help in determining the best time course for return to practice and return to play.
ACKNOWLEDGMENTS
This work was supported by NS37365, NS30308, NS27544, and the Lind Lawrence Foundation.
REFERENCES
- 1.Takahashi H, Manaka S, Sano K. Changes in extracellular potassium concentration in cortex and brain stem during the acute phase of experimental closed head injury. J Neurosurg. 1981;55:708–717. doi: 10.3171/jns.1981.55.5.0708. [DOI] [PubMed] [Google Scholar]
- 2.Hubschmann OR, Kornhauser D. Effects of intraparenchymal hemorrhage on extracellular cortical potassium in experimental head trauma. J Neurosurg. 1983;59:289–293. doi: 10.3171/jns.1983.59.2.0289. [DOI] [PubMed] [Google Scholar]
- 3.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 4.Julian F, Goldman D. The effects of mechanical stimulation on some electrical properties of axons. J Gen Physiol. 1962;46:297–313. doi: 10.1085/jgp.46.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballanyi K, Grafe P, ten Bruggencate G. Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J Physiol. 1987;382:159–174. doi: 10.1113/jphysiol.1987.sp016361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuffler SW. Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci. 1967;168:1–21. doi: 10.1098/rspb.1967.0047. [DOI] [PubMed] [Google Scholar]
- 7.Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987;237:896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Impaired K(+) homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci. 1999;19:8152–8162. doi: 10.1523/JNEUROSCI.19-18-08152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astrup J, Rehncrona S, Siesjo BK. The increase in extracellular potassium concentration in the ischemic brain in relation to the preischemic functional activity and cerebral metabolic rate. Brain Res. 1980;199:161–174. doi: 10.1016/0006-8993(80)90238-3. [DOI] [PubMed] [Google Scholar]
- 10.Hansen AJ. Extracellular potassium concentration in juvenile and adult rat brain cortex during anoxia. Acta Physiol Scand. 1977;99:412–420. doi: 10.1111/j.1748-1716.1977.tb10394.x. [DOI] [PubMed] [Google Scholar]
- 11.Hansen AJ. The extracellular potassium concentration in brain cortex following ischemia in hypo- and hyperglycemic rats. Acta Physiol Scand. 1978;102:324–329. doi: 10.1111/j.1748-1716.1978.tb06079.x. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson C, Kraig RP. The behavior of extracellular ions during spreading depression. In: Zeuthen T, editor. The Application of Ion-Selective Electrodes. New York, NY: Elsevier, North-Holland; 1981. pp. 217–238. [Google Scholar]
- 13.Prince DA, Lux HD, Neher E. Measurement of extracellular potassium activity in cat cortex. Brain Res. 1973;50:489–495. doi: 10.1016/0006-8993(73)90758-0. [DOI] [PubMed] [Google Scholar]
- 14.Sugaya E, Takato M, Noda Y. Neuronal and glial activity during spreading depression in cerebral cortex of cat. J Neurophysiol. 1975;38:822–841. doi: 10.1152/jn.1975.38.4.822. [DOI] [PubMed] [Google Scholar]
- 15.Van Harreveld A. Two mechanisms for spreading depression in the chicken retina. J Neurobiol. 1978;9:419–431. doi: 10.1002/neu.480090602. [DOI] [PubMed] [Google Scholar]
- 16.Somjen GG, Giacchino JL. Potassium and calcium concentrations in interstitial fluid of hippocampal formation during paroxysmal responses. J Neurophysiol. 1985;53:1098–1108. doi: 10.1152/jn.1985.53.4.1098. [DOI] [PubMed] [Google Scholar]
- 17.Bull RJ, Cummins JT. Influence of potassium on the steady-state redox potential of the electron transport chain in slices of rat cerebral cortex and the effect of ouabain. J Neurochem. 1973;21:923–937. doi: 10.1111/j.1471-4159.1973.tb07537.x. [DOI] [PubMed] [Google Scholar]
- 18.Mayevsky A, Chance B. Repetitive patterns of metabolic changes during cortical spreading depression of the awake rat. Brain Res. 1974;65:529–533. doi: 10.1016/0006-8993(74)90243-1. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal M, LaManna J, Yamada S, Younts W, Somjen G. Oxidative metabolism, extracellular potassium and sustained potential shifts in cat spinal cord in situ. Brain Res. 1979;162:113–127. doi: 10.1016/0006-8993(79)90760-1. [DOI] [PubMed] [Google Scholar]
- 20.Shah KR, West M. The effect of concussion on cerebral uptake of 2-deoxy-D-glucose in rat. Neurosci Lett. 1983;40:287–291. doi: 10.1016/0304-3940(83)90053-8. [DOI] [PubMed] [Google Scholar]
- 21.Sunami K, Nakamura T, Ozawa Y, Kubota M, Namba H, Yamaura A. Hypermetabolic state following experimental head injury. Neurosurg Rev. 1989;12(suppl 1):400–411. doi: 10.1007/BF01790682. [DOI] [PubMed] [Google Scholar]
- 22.Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- 23.Samii A, Lee SM, Hovda DA. Delayed increases in glucose utilization following cortical impact injury. Program and abstracts of the Annual Meeting of the Society for Neuroscience; November 7–12, 1998; Los Angeles, CA. Abstract 24, 738. [Google Scholar]
- 24.Ackermann RF, Lear JL. Glycolysis-induced discordance between glucose metabolic rates measured with radiolabeled fluorodeoxyglucose and glucose. J Cereb Blood Flow Metab. 1989;9:774–785. doi: 10.1038/jcbfm.1989.111. [DOI] [PubMed] [Google Scholar]
- 25.Lear JL, Ackermann RF. Why the deoxyglucose method has proven so useful in cerebral activation studies: the unappreciated prevalence of stimulation-induced glycolysis. J Cereb Blood Flow Metab. 1989;9:911–913. doi: 10.1038/jcbfm.1989.128. [DOI] [PubMed] [Google Scholar]
- 26.Corbett RJ, Laptook AR, Nunnally RL, Hassan A, Jackson J. Intracellular pH, lactate, and energy metabolism in neonatal brain during partial ischemia measured in vivo by 31P and 1H nuclear magnetic resonance spectroscopy. J Neurochem. 1988;51:1501–1509. doi: 10.1111/j.1471-4159.1988.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 27.Biros MH, Dimlich RV. Brain lactate during partial global ischemia and reperfusion: effect of pretreatment with dichloroacetate in a rat model. Am J Emerg Med. 1987;5:271–277. doi: 10.1016/0735-6757(87)90349-4. [DOI] [PubMed] [Google Scholar]
- 28.Richards TL, Keniry MA, Weinstein PR, et al. Measurement of lactate accumulation by in vivo proton NMR spectroscopy during global cerebral ischemia in rats. Magn Reson Med. 1987;5:353–357. doi: 10.1002/mrm.1910050406. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson B, Ponten U. Experimental head injury in the rat, part 2: regional brain energy metabolism in concussive trauma. J Neurosurg. 1977;47:252–261. doi: 10.3171/jns.1977.47.2.0252. [DOI] [PubMed] [Google Scholar]
- 30.Yang MS, DeWitt DS, Becker DP, Hayes RL. Regional brain metabolite levels following mild experimental head injury in the cat. J Neurosurg. 1985;63:617–621. doi: 10.3171/jns.1985.63.4.0617. [DOI] [PubMed] [Google Scholar]
- 31.Meyer JS, Kondo A, Nomura F, Sakamoto K, Teraura T. Cerebral hemodynamics and metabolism following experimental head injury. J Neurosurg. 1970;32:304–319. doi: 10.3171/jns.1970.32.3.0304. [DOI] [PubMed] [Google Scholar]
- 32.Nelson SR, Lowry OH, Passonneau JV. Changes in energy reserves in mouse brain associated with compressive head injury. In: Caveness WF, Walker AE, editors. Head Injury. Philadelphia, PA: JB Lippincott; 1966. pp. 444–447. [Google Scholar]
- 33.Nilsson B, Nordstrom CH. Rate of cerebral energy consumption in concussive head injury in the rat. J Neurosurg. 1977;47:274–281. doi: 10.3171/jns.1977.47.2.0274. [DOI] [PubMed] [Google Scholar]
- 34.Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP. Mitochondrial dysfunction after experimental and human brain injury and its possible reversal with a selective N-type calcium channel antagonist (SNX-111) Neurol Res. 1997;19:334–339. doi: 10.1080/01616412.1997.11740821. [DOI] [PubMed] [Google Scholar]
- 35.Xiong Y, Peterson PL, Muizelaar JP, Lee CP. Amelioration of mitochondrial function by a novel antioxidant U-101033E following traumatic brain injury in rats. J Neurotrauma. 1997;14:907–917. doi: 10.1089/neu.1997.14.907. [DOI] [PubMed] [Google Scholar]
- 36.Xiong Y, Peterson PL, Verweij BH, Vinas FC, Muizelaar JP, Lee CP. Mitochondrial dysfunction after experimental traumatic brain injury: combined efficacy of SNX-111 and U-101033E. J Neurotrauma. 1998;15:531–544. doi: 10.1089/neu.1998.15.531. [DOI] [PubMed] [Google Scholar]
- 37.Gardiner M, Smith ML, Kagstrom E, Shohami E, Siesjo BK. Influence of blood glucose concentration on brain lactate accumulation during severe hypoxia and subsequent recovery of brain energy metabolism. J Cereb Blood Flow Metab. 1982;2:429–438. doi: 10.1038/jcbfm.1982.49. [DOI] [PubMed] [Google Scholar]
- 38.Kalimo H, Rehncrona S, Soderfeldt B, Olsson Y, Siesjo BK. Brain lactic acidosis and ischemic cell damage, 2: histopathology. J Cereb Blood Flow Metab. 1981;1:313–327. doi: 10.1038/jcbfm.1981.35. [DOI] [PubMed] [Google Scholar]
- 39.Kalimo H, Rehncrona S, Soderfeldt B. The role of lactic acidosis in the ischemic nerve cell injury. Acta Neuropathol Suppl (Berl) 1981;7:20–22. doi: 10.1007/978-3-642-81553-9_6. [DOI] [PubMed] [Google Scholar]
- 40.Myers RE. A unitary theory of causation of anoxic and hypoxic brain pathology. Adv Neurol. 1979;26:195–213. [PubMed] [Google Scholar]
- 41.Siemkowicz E, Hansen AJ. Clinical restitution following cerebral ischemia in hypo-, normo- and hyperglycemic rats. Acta Neurol Scand. 1978;58:1–8. [PubMed] [Google Scholar]
- 42.Becker DP, Jenkins LW. The pathophysiology of head trauma. In: Miller TA, Rowlands B, editors. The Physiological Basis of Modern Surgical Care. St Louis, MO: Mosby; 1987. pp. 763–788. [Google Scholar]
- 43.Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan XQ, Prough DS, Smith TL, DeWitt DS. The effects of traumatic brain injury on regional cerebral blood flow in rats. J Neurotrauma. 1988;5:289–301. doi: 10.1089/neu.1988.5.289. [DOI] [PubMed] [Google Scholar]
- 45.Yamakami I, McIntosh TK. Effects of traumatic brain injury on regional cerebral blood flow in rats as measured with radiolabeled microspheres. J Cereb Blood Flow Metab. 1989;9:117–124. doi: 10.1038/jcbfm.1989.16. [DOI] [PubMed] [Google Scholar]
- 46.Velarde F, Fisher DT, Hovda DA. Fluid percussion injury induces prolonged changes in cerebral blood flow. J Neurotrauma. 1992;9:402. [Google Scholar]
- 47.Doberstein C, Velarde F, Badie H, Hovda DA. Society for Neuroscience. Anaheim, CA: 1992. Jan 1, Changes in local cerebral blood flow following concussive brain injury. Abstract 18,175. [Google Scholar]
- 48.Ginsberg MD, Zhao W, Alonso OF, Loor-Estades JY, Dietrich WD, Busto R. Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid percussion injury in rats. Am J Physiol. 1997;272(6 pt 2):H2859–H2868. doi: 10.1152/ajpheart.1997.272.6.H2859. [DOI] [PubMed] [Google Scholar]
- 49.Cortez SC, McIntosh TK, Noble LJ. Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res. 1989;482:271–282. doi: 10.1016/0006-8993(89)91190-6. [DOI] [PubMed] [Google Scholar]
- 50.Fineman I, Hovda DA, Smith M, Yoshino A, Becker DP. Concussive brain injury is associated with a prolonged accumulation of calcium: a 45Ca autoradiographic study. Brain Res. 1993;624:94–102. doi: 10.1016/0006-8993(93)90064-t. [DOI] [PubMed] [Google Scholar]
- 51.McIntosh TK. Novel pharmacologic therapies in the treatment of experimental traumatic brain injury: a review. J Neurotrauma. 1993;10:215–261. doi: 10.1089/neu.1993.10.215. [DOI] [PubMed] [Google Scholar]
- 52.Osteen CL, Moore AH, Prins ML, Hovda DA. Age-dependency of 45calcium accumulation following lateral fluid percussion: acute and delayed patterns. J Neurotrauma. 2001;18:141–162. doi: 10.1089/08977150150502587. [DOI] [PubMed] [Google Scholar]
- 53.Samii A, Badie H, Fu K, Luther RR, Hovda DA. Effects of an N-type calcium channel antagonist (SNX 111; Ziconotide) on calcium-45 accumulation following fluid-percussion injury. J Neurotrauma. 1999;16:879–892. doi: 10.1089/neu.1999.16.879. [DOI] [PubMed] [Google Scholar]
- 54.Takizawa S, Matsushima K, Fujita H, Nanri K, Ogawa S, Shinohara Y. A selective N-type calcium channel antagonist reduces extracellular glutamate release and infarct volume in focal cerebral ischemia. J Cereb Blood Flow Metab. 1995;15:611–618. doi: 10.1038/jcbfm.1995.75. [DOI] [PubMed] [Google Scholar]
- 55.Nilsson P, Laursen H, Hillered L, Hansen AJ. Calcium movements in traumatic brain injury: the role of glutamate receptor-operated ion channels. J Cereb Blood Flow Metab. 1996;16:262–270. doi: 10.1097/00004647-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Nadler V, Biegon A, Beit-Yannai E, Adamchik J, Shohami E. 45Ca accumulation in rat brain after closed head injury: attenuation by the novel neuroprotective agent HU-211. Brain Res. 1995;685:1–11. doi: 10.1016/0006-8993(95)00367-y. [DOI] [PubMed] [Google Scholar]
- 57.Hovda DA, Yoshino A, Kawamata T, Katayama Y, Becker DP. Diffuse prolonged depression of cerebral oxidative metabolism following concussive brain injury in the rat: a cytochrome oxidase histochemistry study. Brain Res. 1991;567:1–10. doi: 10.1016/0006-8993(91)91429-5. [DOI] [PubMed] [Google Scholar]
- 58.Bergsneider M, Hovda DA, Lee SM, et al. Dissociation of cerebral glucose metabolism and level of consciousness during the period of metabolic depression following human traumatic brain injury. J Neurotrauma. 2000;17:389–401. doi: 10.1089/neu.2000.17.389. [DOI] [PubMed] [Google Scholar]
- 59.Vink R, McIntosh TK, Demediuk P, Faden AI. Decrease in total and free magnesium concentration following traumatic brain injury in rats. Biochem Biophys Res Commun. 1987;149:594–599. doi: 10.1016/0006-291x(87)90409-8. [DOI] [PubMed] [Google Scholar]
- 60.Vink R, McIntosh TK, Weiner MW, Faden AI. Effects of traumatic brain injury on cerebral high-energy phosphates and pH: a 31P magnetic resonance spectroscopy study. J Cereb Blood Flow Metab. 1987;7:563–571. doi: 10.1038/jcbfm.1987.106. [DOI] [PubMed] [Google Scholar]
- 61.Vink R, Faden AI, McIntosh TK. Changes in cellular bioenergetic state following graded traumatic brain injury in rats: determination by phosphorus 31 magnetic resonance spectroscopy. J Neurotrauma. 1988;5:315–330. doi: 10.1089/neu.1988.5.315. [DOI] [PubMed] [Google Scholar]
- 62.Vink R, McIntosh TK. Pharmacological and physiological effects of magnesium on experimental traumatic brain injury. Magnes Res. 1990;3:163–169. [PubMed] [Google Scholar]
- 63.McIntosh TK, Faden AI, Yamakami I, Vink R. Magnesium deficiency exacerbates and pretreatment improves outcome following traumatic brain injury in rats: 31P magnetic resonance spectroscopy and behavioral studies. J Neurotrauma. 1988;5:17–31. doi: 10.1089/neu.1988.5.17. [DOI] [PubMed] [Google Scholar]
- 64.Pettus EH, Christman CW, Giebel ML, Povlishock JT. Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. J Neurotrauma. 1994;11:507–522. doi: 10.1089/neu.1994.11.507. [DOI] [PubMed] [Google Scholar]
- 65.Povlishock JT, Pettus EH. Traumatically induced axonal damage: evidence for enduring changes in axolemmal permeability with associated cytoskeletal change. Acta Neurochir Suppl (Wien) 1996;66:81–86. doi: 10.1007/978-3-7091-9465-2_15. [DOI] [PubMed] [Google Scholar]
- 66.Mata M, Staple J, Fink DJ. Changes in intra-axonal calcium distribution following nerve crush. J Neurobiol. 1986;17:449–467. doi: 10.1002/neu.480170508. [DOI] [PubMed] [Google Scholar]
- 67.Maxwell WL, McCreath BJ, Graham DI, Gennarelli TA. Cytochemical evidence for redistribution of membrane pump calcium-ATPase and ecto-Ca-ATPase activity, and calcium influx in myelinated nerve fibres of the optic nerve after stretch injury. J Neurocytol. 1995;24:925–942. doi: 10.1007/BF01215643. [DOI] [PubMed] [Google Scholar]
- 68.Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakamura Y, Takeda M, Angelides KJ, Tanaka T, Tada K, Nishimura T. Effect of phosphorylation on 68 KDa neurofilament subunit protein assembly by the cyclic AMP dependent protein kinase in vitro. Biochem Biophys Res Commun. 1990;169:744–750. doi: 10.1016/0006-291x(90)90394-3. [DOI] [PubMed] [Google Scholar]
- 70.Nixon RA. The regulation of neurofilament protein dynamics by phosphorylation: clues to neurofibrillary pathobiology. Brain Pathol. 1993;3:29–38. doi: 10.1111/j.1750-3639.1993.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 71.Johnson GV, Greenwood JA, Costello AC, Troncoso JC. The regulatory role of calmodulin in the proteolysis of individual neurofilament proteins by calpain. Neurochem Res. 1991;16:869–873. doi: 10.1007/BF00965535. [DOI] [PubMed] [Google Scholar]
- 72.Pettus EH, Povlishock JT. Characterization of a distinct set of intra-axonal ultrastructural changes associated with traumatically induced alteration in axolemmal permeability. Brain Res. 1996;722:1–11. doi: 10.1016/0006-8993(96)00113-8. [DOI] [PubMed] [Google Scholar]
- 73.Maxwell WL, Graham DI. Loss of axonal microtubules and neurofilaments after stretch-injury to guinea pig optic nerve fibers. J Neurotrauma. 1997;14:603–614. doi: 10.1089/neu.1997.14.603. [DOI] [PubMed] [Google Scholar]
- 74.Povlishock JT, Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma. 1995;12:555–564. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- 75.Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- 76.Farooqui AA, Horrocks LA. Excitatory amino acid receptors, neural membrane phospholipid metabolism and neurological disorders. Brain Res Brain Res Rev. 1991;16:171–191. doi: 10.1016/0165-0173(91)90004-r. [DOI] [PubMed] [Google Scholar]
- 77.Kampfl A, Posmantur RM, Zhao X, Schmutzhard E, Clifton GL, Hayes RL. Mechanisms of calpain proteolysis following traumatic brain injury, implications for pathology and therapy: a review and update. J Neurotrauma. 1997;14:121–134. doi: 10.1089/neu.1997.14.121. [DOI] [PubMed] [Google Scholar]
- 78.Roberts-Lewis JM, Siman R. Spectrin proteolysis in the hippocampus: a biochemical marker for neuronal injury and neuroprotection. Ann N Y Acad Sci. 1993;679:78–86. doi: 10.1111/j.1749-6632.1993.tb18290.x. [DOI] [PubMed] [Google Scholar]
- 79.Verity MA. Ca(2+)-dependent processes as mediators of neurotoxicity. Neurotoxicology. 1992;13:139–147. [PubMed] [Google Scholar]
- 80.Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia, part II: mechanisms of damage and treatment. J Neurosurg. 1992;77:337–354. doi: 10.3171/jns.1992.77.3.0337. [DOI] [PubMed] [Google Scholar]
- 81.Iwasaki Y, Yamamoto H, Iizuka H, Yamamoto T, Konno H. Suppression of neurofilament degradation by protease inhibitors in experimental spinal cord injury. Brain Res. 1987;406:99–104. doi: 10.1016/0006-8993(87)90773-6. [DOI] [PubMed] [Google Scholar]
- 82.Morgan JI, Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- 83.Miller LP, Lyeth BG, Jenkins LW, et al. Excitatory amino acid receptor subtype binding following traumatic brain injury. Brain Res. 1990;526:103–107. doi: 10.1016/0006-8993(90)90254-9. [DOI] [PubMed] [Google Scholar]
- 84.Giza CC, Lee SM, Kremen TJ, Hovda DA. Decreased N-methyl D-aspartate receptor (NMDAR) activity after developmental fluid percussion injury (FPI) demonstrated by changes in subunit composition [abstract] Restorative Neurol Neurosci. 2000;16:170. [Google Scholar]
- 85.Osteen CL, Giza CC, Hovda DA. Changes in N-methyl D-aspartate receptor (NMDAR) number and subunit composition after fluid percussion (FP) injury appear to prepare the hippocampus for neuroplasticity in adult rats [abstract] Restorative Neurol Neurosci. 2000;16:210. [Google Scholar]
- 86.Feeney DM, Sutton RL, Boyeson MG. The locus coeruleus and cerebral metabolism: recovery of function after cortical injury. Physiol Psychol. 1985;13:197–203. [Google Scholar]
- 87.Pappius HM. Significance of biogenic amines in functional disturbances resulting from brain injury. Metab Brain Dis. 1988;3:303–310. doi: 10.1007/BF00999542. [DOI] [PubMed] [Google Scholar]
- 88.Gorman LK, Fu K, Hovda DA, Murray M, Traystman RJ. Effects of traumatic brain injury on the cholinergic system in the rat. J Neurotrauma. 1996;13:457–463. doi: 10.1089/neu.1996.13.457. [DOI] [PubMed] [Google Scholar]
- 89.D'Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Res. 1998;786:64–79. doi: 10.1016/s0006-8993(97)01412-1. [DOI] [PubMed] [Google Scholar]
- 90.Sick TJ, Perez-Pinzon MA, Feng ZZ. Impaired expression of long-term potentiation in hippocampal slices 4 and 48 h following mild fluid-percussion brain injury in vivo. Brain Res. 1998;785:287–292. doi: 10.1016/s0006-8993(97)01418-2. [DOI] [PubMed] [Google Scholar]
- 91.Sanders MJ, Sick TJ, Perez-Pinzon MA, Dietrich WD, Green EJ. Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res. 2000;861:69–76. doi: 10.1016/s0006-8993(00)01986-7. [DOI] [PubMed] [Google Scholar]
- 92.Schmidt RH, Grady MS. Loss of forebrain cholinergic neurons following fluid-percussion injury: implications for cognitive impairment in closed head injury. J Neurosurg. 1995;83:496–502. doi: 10.3171/jns.1995.83.3.0496. [DOI] [PubMed] [Google Scholar]
- 93.Hepler DJ, Olton DS, Wenk GL, Coyle JT. Lesions in nucleus basalis magnocellularis and medial septal area of rats produce qualitatively similar memory impairments. J Neurosci. 1985;5:866–873. doi: 10.1523/JNEUROSCI.05-04-00866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyamoto M, Kato J, Narumi S, Nagaoka A. Characteristics of memory impairment following lesioning of the basal forebrain and medial septal nucleus in rats. Brain Res. 1987;419:19–31. doi: 10.1016/0006-8993(87)90564-6. [DOI] [PubMed] [Google Scholar]
- 95.Toth Z, Hollrigel GS, Gorcs T, Soltesz I. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J Neurosci. 1997;17:8106–8117. doi: 10.1523/JNEUROSCI.17-21-08106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee SM, Smith ML, Hovda DA, Becker DP. Society for Neuroscience. San Diego, CA: 1995. Nov 8–11, Concussive brain injury results in chronic vulnerability of post-traumatic seizures. Abstract 21,762. [Google Scholar]
- 97.Prins ML, Lee SM, Cheng CL, Becker DP, Hovda DA. Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res Dev Brain Res. 1996;95:272–282. doi: 10.1016/0165-3806(96)00098-3. [DOI] [PubMed] [Google Scholar]
- 98.Prins ML, Hovda DA. Traumatic brain injury in the developing rat: effects of maturation on Morris water maze acquisition. J Neurotrauma. 1998;15:799–811. doi: 10.1089/neu.1998.15.799. [DOI] [PubMed] [Google Scholar]
- 99.Adelson PD, Dixon CE, Robichaud P, Kochanek PM. Motor and cognitive functional deficits following diffuse traumatic brain injury in the immature rat. J Neurotrauma. 1997;14:99–108. doi: 10.1089/neu.1997.14.99. [DOI] [PubMed] [Google Scholar]
- 100.Pickles W. Acute general edema of the brain in children with head injuries. N Engl J Med. 1950;242:607–611. doi: 10.1056/NEJM195004202421602. [DOI] [PubMed] [Google Scholar]
- 101.Bruce DA, Alavi A, Bilaniuk L, Dolinskas C, Obrist W, Uzzell B. Diffuse cerebral swelling following head injuries in children: the syndrome of “malignant brain edema.”. J Neurosurg. 1981;54:170–178. doi: 10.3171/jns.1981.54.2.0170. [DOI] [PubMed] [Google Scholar]
- 102.Aldrich EF, Eisenberg HM, Saydjari C, et al. Diffuse brain swelling in severely head-injured children: a report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1992;76:450–454. doi: 10.3171/jns.1992.76.3.0450. [DOI] [PubMed] [Google Scholar]
- 103.Klonoff H, Low MD, Clark C. Head injuries in children: a prospective five year follow-up. J Neurol Neurosurg Psychiatry. 1977;40:1211–1219. doi: 10.1136/jnnp.40.12.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Levin HS, Eisenberg HM, Wigg NR, Kobayashi K. Memory and intellectual ability after head injury in children and adolescents. Neurosurgery. 1982;11:668–673. doi: 10.1227/00006123-198211000-00009. [DOI] [PubMed] [Google Scholar]
- 105.Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 106.Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and anatomical plasticity of brain. Science. 1964;164:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- 107.Greenough WT, Volkmar FR, Juraska JM. Effects of rearing complexity on dendritic branching in frontolateral and temporal cortex of the rat. Exp Neurol. 1973;41:371–378. doi: 10.1016/0014-4886(73)90278-1. [DOI] [PubMed] [Google Scholar]
- 108.Tees RC, Buhrmann K, Hanley J. The effect of early experience on water maze spatial learning and memory in rats. Dev Psychobiol. 1990;23:427–439. doi: 10.1002/dev.420230505. [DOI] [PubMed] [Google Scholar]
- 109.Fineman I, Giza CC, Nahed BV, Lee SM, Hovda DA. Inhibition of neocortical plasticity during development by a moderate concussive brain injury. J Neurotrauma. 2000;17:739–749. doi: 10.1089/neu.2000.17.739. [DOI] [PubMed] [Google Scholar]
- 110.Schallert T, Kozlowski DA, Humm JL, Cocke RR. Use-dependent structural events in recovery of function. Adv Neurol. 1997;73:229–238. [PubMed] [Google Scholar]
- 111.Kozlowski DA, James DC, Schallert T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci. 1996;16:4776–4786. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Humm JL, Kozlowski DA, James DC, Gotts JE, Schallert T. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res. 1998;783:286–292. doi: 10.1016/s0006-8993(97)01356-5. [DOI] [PubMed] [Google Scholar]
- 113.Jenkins LW, Marmarou A, Lewelt W, Becker DP. Increased vulnerability of the traumatized brain to early ischemia. In: Baethmann A, Go GK, editors. Mechanisms of Secondary Brain Damage. New York, NY: Plenum Press; 1986. pp. 273–282. [Google Scholar]
- 114.Fu K, Smith ML, Thomas S, Hovda DA. Cerebral concussion produces a state of vulnerability lasting for as long as 5 hours [abstract] J Neurotrauma. 1992;9:59. [Google Scholar]
- 115.Badie H, Hovda DA, Becker DP. Glial fibrillary acidic protein (GFAP) expression following concussive brain injury: a quantitative study of the effects of a second insult [abstract] J Neurotrauma. 1992;9:56. [Google Scholar]
- 116.Allen GV, Gerami D, Esser MJ. Conditioning effects of repetitive mild neurotrauma on motor function in an animal model of focal brain injury. Neuroscience. 2000;99:93–105. doi: 10.1016/s0306-4522(00)00185-8. [DOI] [PubMed] [Google Scholar]