Abstract
Objective:
To identify the types of injuries the human brain incurs as a result of traumatic forces applied to the cranium. In athletic events and endeavors, the full spectrum of intracranial hemorrhages in various compartments, raised intracranial pressure, and diffuse nonhemorrhagic damage may be seen. In this review, we describe these serious injuries and the more common mild traumatic brain injury in their clinical presentations and relate concussion classification to the overall picture of traumatic brain injury.
Methods:
Our cumulative experience with athletic injuries, both at the catastrophic and mild traumatic brain injury levels, has led us to a management paradigm that serves to guide us in the classification and treatment of these athletes.
Discussion:
The occurrence of intracranial injuries in sports has now been well documented. Intracranial hematomas (epidural, subdural, and parenchymal) and cerebral contusions can result from head injury. Many patients sustain a diffuse brain injury, resulting in elevated intracranial pressures, without a blood clot or mass lesion. The classification of concussion and the use of concussion guidelines are not uniform. However, the major emphases are agreed upon: the close and careful scrutiny of the athlete, an expeditious but reliable neurologic examination, and proper on-field management. Return-to-play decisions are based on many factors that affect normal functioning, both on and off the playing field.
Conclusions:
Sufficient knowledge now exists to allow us to carefully evaluate the injured athlete, to place him or her in the management scheme to minimize the potential for permanent cerebral dysfunction, and to know when the athlete can safely return to contact sport participation.
Keywords: diffuse brain injury, hematoma
As the number of participants in recreational and organized sporting events continues to increase, we are faced with an expanding interest in understanding the effects of mild traumatic brain injury (MTBI). Concussion, as MTBI is commonly termed, is a concern in every sport that has head impact or collision as a possible consequence. Paramount to an appreciation for the seriousness and potential consequences of concussion is the concept that it exists as a pathophysiologic entity along a spectrum of injury, ranging from mild concussion to severe, diffuse injuries.1,2 Associated severe cognitive, memory, and motor deficits persist among survivors of severe, diffuse brain injury, and the mortality rate is greater than 50%.3–5
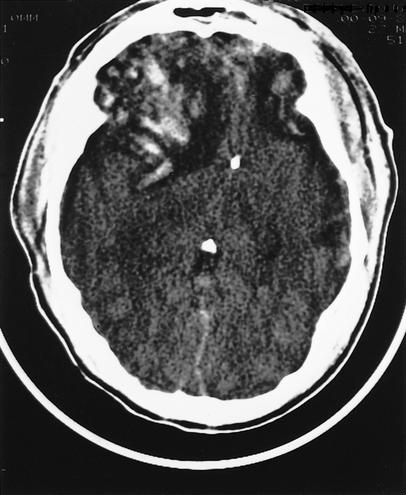
Diffuse brain injury differs from focal brain injury in that a global insult results in widespread neuronal dysfunction. Diffuse brain injury occurs along a continuum of diffuse axonal injury (DAI), represented by shearing of white-matter fiber tracts as they course from the cortex to the midbrain and brain stem, producing characteristic radiographic findings (Figures 1 and 2).6 Rotational energy forces lead to disruption of axons along the neuroaxis, marked by small areas of hemorrhage.7 Disruption of axon fibers projecting within the cerebrum and brain stem has been documented in autopsy studies in patients with MTBI.3,4
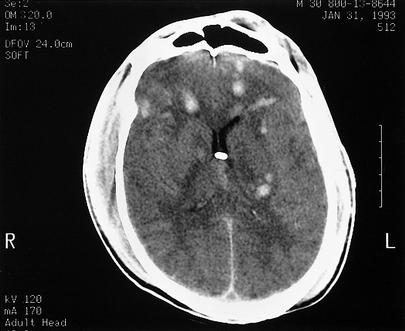
Figure 1.
Computed tomography (CT) scan showing diffuse axonal injury in a 30-year-old male victim of a motor vehicle accident. Patients with diffuse axonal injury classically are unconscious from the moment of impact, and impairment is often worse than in patients with other primary injury (contusions, intracerebral hemorrhage, subdural hematoma, epidural hematoma). Diffuse axonal injury characteristically appears as multiple small, focal scattered lesions throughout the white matter, usually at gray-white junctions, sparing the cortex. CT and magnetic resonance imaging (MRI) underestimate the true extent of injury.
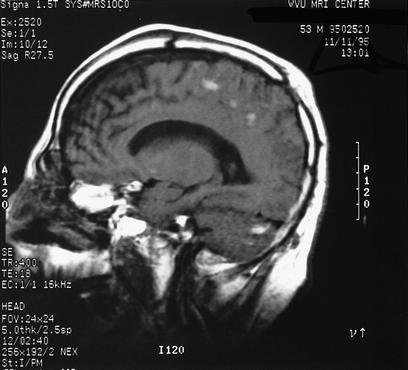
Figure 2.
MRI scan in a comatose patient with closed head injury showing diffuse axonal injury changes. While large hematomas with mass effect are ordinarily not seen, diffuse axonal injury represents widespread shearing of axons and multiple areas of injury.
As kinetic energy is applied to the human cranium, both acceleration-deceleration and rotational mechanisms occur. Acceleration-deceleration injury, also considered translational (linear) impact, usually results when the subject's body and head are traveling at a particular speed and strike a solid object. Similarly, a head at rest may be struck by a moving object. The resultant injury causes linear, tensile, and compressive strains that disrupt the cerebral anatomy and cytoarchitecture. Rotational (angular) movements also take place, secondary to the fixation of the brain at the foramen magnum and craniospinal junction and the relative tethering of the midbrain as it passes through the tentorial hiatus. Energy directed to the head may cause transmission of force in a rotatory direction, often producing diffuse brain injury with shearing of the white-matter fiber tracts.3
Although it is possible for combinations of biomechanical factors to coexist during head injury, observation has shown that one mechanism is usually predominant. In wrestling, the takedown or activity associated with this maneuver usually causes MTBI.8 Biomechanical studies concerning athletic injuries have suggested that angular head accelerations have a higher incidence of injury.9 Acceleration-deceleration energy forces are more likely in contact sports, especially football, ice hockey, and boxing. These can happen within the framework of the contest rules and often within a single event. Blocking and tackling, checking, jabs, cross-punches, and many other techniques predominately deliver acceleration-deceleration or linear energy vectors to the contestant. In contrast, mechanisms exemplified by the boxing hook punch impart rotatory forces to the mandible and cranium. The rotatory component of the cranial or mandibular impacts often contributes to the forces that cause loss of consciousness (LOC), providing the etiology for many athletic cerebral concussions.
In addition, forces applied to the head in contact sports have also been described as impact or impulsive loading. Resulting from a rapidly applied energy input, impact loading is a direct blow to the cranium occurring over less than 200 milliseconds. This can cause skull deformation and energy shock-wave propagation through the skull and brain, resulting in underlying cerebral injury. In contrast, when the head is placed in motion and suddenly accelerated or decelerated as a result of either an impact to another part of the body or as a secondary response to a direct impact, impulsive loading occurs. This mechanism causes compressive, shear, and tensile stresses to the brain, leading to more diffuse or remote injuries. It is more often seen with rotational energy inputs and is less effectively prevented by protective headgear, which is better able to dissipate impact-loading energy input.10
Presence of the normal state of consciousness signifies that the person is awake and alert with the ability to interact with the environment. A normal level of consciousness depends on a complex interaction of cortical, subcortical, and brain stem nuclei. Alteration of the state of consciousness occurs when the integrity of this neurophysiologic functional unit has been interrupted. The reticular activating system extending through the brain stem must interact with the hypothalamus and cerebral hemispheres in a normal feedback-loop mechanism for consciousness to be maintained. Any alteration of this circuitry and feedback, therefore, produces a change in the state of consciousness. The major focus of categorizing concussions in sports in the past has centered on the occurrence of LOC. We now appreciate that other and ongoing expressions of cerebral dysfunction, such as memory and cognitive dysfunction, are important and likely predictive. This is especially true when considering only the very brief period of LOC, which may not be as indicative of a serious athletic injury.11,12
In addition to the biomechanics, recent experimenters have elucidated the biomechanical abnormalities that represent concussion. Activation of the glycolytic process as a result of cellular requirements to maintain ionic gradients is believed to be present in concussion. Large increases occur in extracellular potassium concentrations through voltage-gated potassium channels.13–15 Neurotransmitters, in particular glutamate, appear to play a significant role in opening ionic channels after even MTBI. A quantitative rise in glucose utilization, in an effort to correct ionic perturbations in transmembrane potentials, is seen in newer concussion models.16,17 Metabolic dysfunction after concussive injury produces a period of neuronal vulnerability in which there is both higher demand for glucose to correct ionic movements and a paradoxical reduction in cerebral blood flow, the latter influenced by calcium movements. The rise in glycolytic energy requirements has been shown both experimentally and clinically to be present within the first several days after concussive brain injury.17
Experimental concussion models have demonstrated transient cerebral ischemia, edema, widespread neuronal depolarization from release of acetylcholine, and the shearing of neurons and nerve fibers (particularly in the brain stem) as potential explanations for alterations of mental status after closed head injury. While no gross neuropathologic changes are consistently demonstrated in experimental concussion, disruption of multiple neurons and their connections, along with scattered capillary damage in the brain stem reticular formation, has been reported.17,18
TRAUMATIC INTRACRANIAL LESIONS
Epidural Hematoma
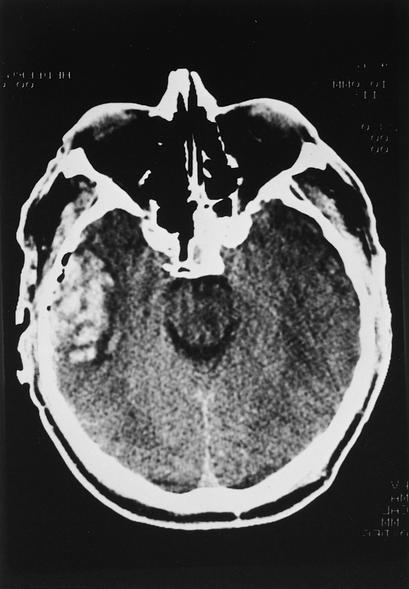
Epidural hematoma is an accumulation of blood between the dura and skull. The dura becomes detached and dissects to the point of dural attachment to the overlying cranium. Hemorrhage occurs beneath the skull and outside the dura, resulting in the classic computed tomography (CT) appearance of a biconvex or lenticular shape of the hematoma (Figure 3). Epidural hematoma is caused by head impact, usually of the acceleration-deceleration type, and can result in inward deformity, leading to dural detachment from the inner table of the skull. Most patients with an epidural hematoma have a skull fracture, which leads to laceration of the middle meningeal artery or vein. In addition, bleeding can occur from the actual bone fragments or the diploic space, leading to collection of blood in the epidural location.
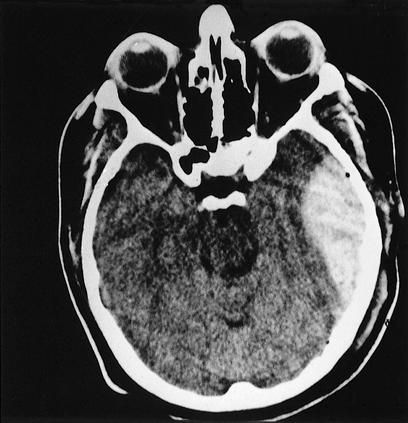
Figure 3.
CT scan showing epidural hematoma in the left middle cranial fossa with significant mass effect. The lenticular or biconvex shape is typical, indicative of its extra-axial location.
Characteristic of epidural hematoma is an isolated injury to the skull, dura, and dural vessels that leads to hematoma formation. In most of these acceleration-deceleration injuries, the skull has sustained the major impact forces and absorbed the resultant kinetic energy. The heavy force delivered to the cranium and transmitted to the brain often disrupts the state of consciousness. In contrast with other injuries, such as subdural hematoma, in which the brain often sustains a primary and major injury, epidural hematoma is often not associated with primary brain injury.
Another important distinguishing feature of this clinical entity is a lucid interval. The lucid interval occurs when a substantial blow has been transmitted to the cranium and causes the person to lose consciousness. The patient may subsequently appear asymptomatic and have a normal neurologic examination. The problem arises when an injury to the skull or dural vessels or both leads to a slow accumulation of blood in the epidural space. This hematoma outside the brain may remain relatively asymptomatic until it reaches a critically large size and compresses the underlying brain.19 The compression can be transmitted to the brain stem and rapidly progress to neurologic dysfunction, brain herniation, and possibly death.20 Any patient or athlete who has sustained a significant head impact should be observed in the awake state and not allowed to retire for sleep until the longer lasting effects of the head impact are known. Any patient with a significant LOC (minutes) or neurologic abnormality should have a more thorough medical evaluation, including CT scanning. The clinical manifestations of epidural hematoma depend on the type and amount of energy transferred, the time course of the hematoma formation, and the presence of simultaneous brain injuries. Often, the size of the hematoma determines the clinical effects. In addition to a lucid interval, patients with an epidural hematoma may present with no LOC, persistent unconsciousness, or any variation of these features.21
Subdural Hematoma
This form of intracranial hemorrhage has been divided into acute subdural hematoma, which presents within 48 to 72 hours after injury, and chronic subdural hematoma, which occurs in a later time frame with more variable clinical manifestations. An acute subdural hematoma is the most common major head injury and is associated with severe neurologic disability and death in many patients. Acute subdural hematoma results from bleeding within the subdural space as a result of stretching and tearing of the subdural veins. These veins drain from the cerebral surface and connect to the dura or dural sinuses. In addition, the bone irregularities of the middle cranial fossa, sphenoid bone, and frontal fossa form a rough surface over which inferior cortical surface contusions can form, resulting in hemorrhage in the subdural space.
A subdural hematoma may occur as an isolated collection of blood within the subdural space or as a more complicated hematoma associated with brain parenchymal injury. Many patients with complicated acute subdural hematomas sustain diffuse irreversible brain damage and do not improve after evacuation of the hematoma, the latter representing an epiphenomenon in the injury process. The outcome of subdural hematoma is thus often influenced by the extent of the concomitant parenchymal brain injury more than the subdural hematoma collection per se.22
The clinical presentation of any patient, including an athlete, with acute subdural hematoma can vary and includes those who are awake and alert with no focal neurologic deficits, but typically patients with any sizable acute subdural hematoma have a significant neurologic deficit (Figure 4). This may consist of alteration of consciousness, often to a state of coma or major focal neurologic deficit. Skull fracture is much less commonly associated with subdural hematoma than with epidural hematoma. Football players with 2 mild concussions without LOC, separated by 7 and 10 days, sustained acute subdural hematomas.23
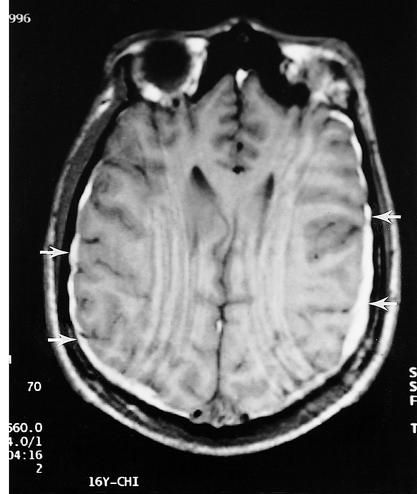
Figure 4.
MRI of a high school football player with persistent headaches and poor school performance. Although the CT scan was normal, the MRI demonstrated small bilateral subdural hematomas, thus documenting the presence of an injury.
A chronic subdural hematoma is defined as a hematoma present 3 weeks or more after a traumatic injury. The pathogenesis of chronic subdural hematoma involves an injury that results in bleeding into the subdural space. The initial hemorrhage may be a small amount that fails to generate significant brain compression. However, bleeding or oozing of blood into the subdural space may continue.24 After 1 week, a chronic subdural hematoma involves infiltration of fibroblasts to organize into an outer membrane. Subsequently, an inner membrane may form, and this encapsulated hematoma may become a dynamic osmotic membrane that interacts with the production and absorption of cerebrospinal fluid. Effusion of protein may occur, setting up an active process within the membrane.25
The diagnosis of chronic subdural hematoma is often difficult because of the protean clinical manifestations. Patients may have clinical symptoms suggestive of increased intracranial pressure, mental disturbance such as personality change or even dementia, symptoms with focal transient neurologic deficits similar to transient ischemic attacks, a meningeal syndrome with nuchal rigidity and photophobia, a clinical course with a slow progression of neurologic signs reminiscent of cerebral neoplasm, or a progressive and severe headache syndrome only.26 Although not common in athletes, chronic subdural hematoma must always be the differential diagnosis, especially in those presenting with a remote history of head impact. The diagnosis is confirmed by CT scanning demonstrating the extra-axial low-density fluid collection in the subdural space.
Intracerebral Hemorrhage
A cerebral contusion is a heterogeneous zone of brain damage that consists of hemorrhage, cerebral infarction, necrosis, and edema. Cerebral contusion is a frequent sequela of head injury and in some studies represents the most common traumatic lesion of the brain visualized on radiographic evaluation. Contusions occur most often as a result of acceleration-deceleration mechanisms from the inward deformation of the skull at the impact site. This results in transient compression of the brain against the skull and the focal area of parenchymal injury. This energy is conducted to the underlying brain, resulting in cerebral contusion, the degree of which depends on the energy transmitted, the area of contact, the involved area of the cranium, and other factors (Figure 5).27
Figure 5.
CT scan in a patient with bifrontal (right greater than left) hemorrhagic contusions. This patient presented in a coma after an acceleration-deceleration injury and did not undergo surgery but instead was managed for increased intracranial pressure.
Contusions can vary from small, localized areas of injury to large, extensive areas of involvement. A cerebral contusion injury can evolve over hours and days after the injury. Multiple small areas of contusions may coalesce into a large area resembling a lesion, more accurately termed intraparenchymal hemorrhage. Injuries remote from the site of cranial impact may also occur. The direct, or coup, lesion results from injury at the impact site, and the remote, or contrecoup, lesion occurs as the opposite side of the brain rebounds against the skull or because of vacuum phenomena existing within the parenchyma at that location. The contrecoup lesion results in a hemorrhagic lesion in the cerebral tissue directly opposite the impact site, typically at the inferior surfaces of the frontal and temporal lobes. Contusions are often multiple and are frequently associated with other extra-axial and intra-axial hemorrhagic lesions.
The clinical course of patients with cerebral contusion varies greatly, depending on the location, number, and extent of the hemorrhagic contusion lesions. The patient may present with essentially normal function or may experience any type of neurologic deterioration, including coma. Frequently, behavioral or mental status changes exist due to involvement of the frontal or temporal lobes. The diagnosis of cerebral contusion is firmly established by CT scanning, which is also useful for following patients as the lesions evolve throughout their clinical course (Figure 6).
Figure 6.
CT scan showing right temporal lobe contusions. These contusions tend to enlarge and lead to brain swelling.
Intracerebral hematoma is a parenchymatous hemorrhage that is often similar in pathophysiology and radiographic appearance to a cerebral contusion. An intracerebral hematoma represents a localized collection of blood within the brain. The distinction between a hemorrhagic contusion and intraparenchymal hematoma depends upon the latter being recognized as a confluent area of homogeneous bleeding within the brain. Intracerebral hematomas usually present with a focal neurologic deficit but may progress to further neurologic deterioration, including coma and death resulting from brain herniation syndromes. This diagnosis is readily made by CT scanning, which shows a hyperdense, localized collection of blood. Intracerebral hematomas have been, along with subdural hematomas, the most common cause of sport-related lethal brain injuries.
Another entity, delayed traumatic intracerebral hematoma, is a clot that forms hours to days after the initial trauma. Although most frequently seen in the older population, it must always be borne in mind when evaluating and attending to any patient who has sustained a significant head impact. The athlete is also at risk because these hematomas are seen more commonly when there has been rotational head trauma. Delayed traumatic hematomas are believed to be due to later bleeding into an already contused region of the brain, to vascular injury, or to the development of a coagulopathy.
Finally, there are severe brain injuries in which the patients do not sustain any form of hematoma or mass effect but instead have a global or diffuse brain insult. This often results in widespread cerebral edema and elevated intracranial pressure (ICP), similar to the pathophysiologic response seen in the second-impact syndrome. In the absence of an operable, space-occupying lesion, the patient is often treated for increased ICP with online ICP monitoring. This is performed by placing a catheter in the brain parenchyma or ventricles, providing a constant measure of ICP. The cerebral perfusion pressure is defined as the mean systemic blood pressure minus the ICP and should be kept at 50 to 70 mm Hg or higher.
Cerebral Concussion
Concussion, or MTBI, is the most common form of head injury seen in athletes. The Centers for Disease Control28 recently proclaimed that approximately 300 000 cases of MTBI occur annually in the United States from athletic activities. Powell and Barber-Foss8 estimated 62 000 cases of concussion in American high school sports annually.
The classical cerebral concussion is defined as a posttraumatic state that results in LOC, with the patient regaining full return of consciousness within 24 hours. Clinically, concussion has often been referred to as physiologic without anatomical disruption in the brain. Newer research has shown both anatomic and biochemical markers of definitive injury secondary to MTBI. Concussion has also been defined as an immediate and transient impairment of neural function, such as alteration of consciousness, disturbance of vision or equilibrium, and other similar symptoms. We now appreciate that concussion symptoms are related to either cerebral cortical dysfunction, such as confusion, disorientation, memory, or information-processing abnormalities, and others, or to brain stem abnormalities. Examples of the latter include the unconscious state, visual or auditory symptoms, nausea and vomiting, ataxia, and incoordination. Some of these patient complaints may also relate to injury to and dysfunction of the inner ear or vestibular mechanism. Sleep disturbances are common and often contribute greatly to the symptom complex.
We have gained a greater appreciation for the importance of having accurate methods for classifying MTBI. This recognition has led to the development of several different classification schemes, unfortunately leading to some uncertainty at times over nomenclature. One must keep in mind that all scales thus far published were formulated based on experiential theory and not on scientifically proven research. No prospective, randomized clinical trial has established any particular concussion classification system as superior or proven. Rather, based on years of experience and observation, these guidelines have been employed as our best and most accurate means of currently categorizing athletic MTBI. The National Athletic Trainers' Association has not adapted or endorsed any one grading scale or set of return-to-play guidelines, nor have most organizations. The best grading scale and return-to-play guidelines will be based on scientific evidence, but most importantly, a systematic evaluation and management scheme must be followed.
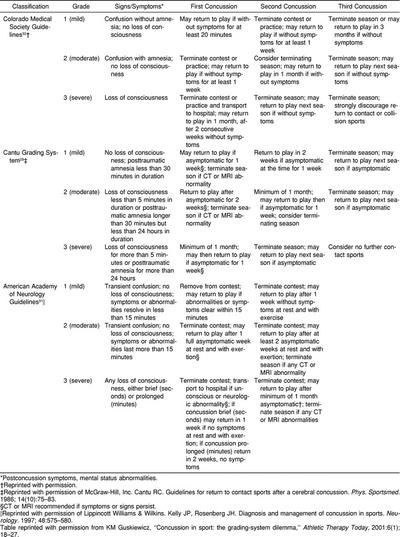
During the last 2 decades, no fewer than 8 classification systems have been proposed for management of sport-related concussions.4,29–32 While disagreement and differences existed regarding terminology and symptom priority, these efforts have led to the recognition of the importance of standardization in the care of the concussed athlete. In recent years, 3 concussion classification schemes have been accepted in widespread application. In 1986, Cantu29 proposed a concussion grading scale in which the persistence of memory disturbance, termed amnesia, is given greater emphasis. The Colorado32 guidelines and the American Academy of Neurology (AAN)31 practice parameters similarly place any athlete with any period of LOC, no matter how brief, within the most severe grade of concussion (Table).
Concussion Management
CONCUSSION CLASSIFICATION
A mild concussion (grade 1) is most commonly seen in athletes. The Colorado guidelines define a grade 1 concussion as no LOC, with confusion being the hallmark sign.32,33 The Cantu definition29 of a mild concussion is one without LOC, with confusion alone or a brief (less than 30 minutes) period of amnesia. The AAN practice parameters31 classify a grade 1 concussion as having no LOC and mental status abnormalities that resolve in less than 15 minutes. This type of concussion is not infrequent in football games, occurring in at least 1 player in nearly every game. If thoroughly searched for, a grade 1 concussion is often found. It may be stated by nonmedical personnel that the player was “dinged.” The athlete, who is awake and alert, may be able to function unnoticed during the course of the athletic contest. If significant disorientation, confusion, memory disturbance, dizziness, headache, or any neurologic abnormality persists after the 15-minute observation period, the athlete has more than a mild concussion. We should remember that concussion may be present and significant without the person's sustaining a LOC. Ommaya and Gennarelli4 showed in their animal model that 3 of 6 grades of concussion did not involve LOC. They postulated that, unless shearing forces reached the reticular activating system within the midbrain and brain stem, cortical and subcortical structures could be affected to produce amnesia and confusion but not LOC.
The Colorado guidelines classify a moderate or grade 2 concussion as associated with the development of amnesia either initially or during the period of observation; there is no LOC.32 The athlete is removed from competition and not allowed to return. Cantu29 defined the moderate concussion as less than 5 minutes of unconsciousness or posttraumatic amnesia for longer than 30 minutes but less than 24 hours' duration, while the AAN system31 specified no LOC but only mental status changes lasting longer than 15 minutes.
A severe or grade 3 concussion is defined as a player having LOC by the Colorado and AAN guidelines.31,32 Cantu29 termed a severe or grade 3 concussion as one having greater than a 5-minute period of unconsciousness or 24 hours of posttraumatic amnesia. This patient may require emergent transport to the nearest hospital facility with CT scanning and, if indicated, consideration should be given for neurosurgical consultation. The possibility of concomitant cervical spine injury must always be considered in an unconscious patient and transport performed with cervical immobilization and maintenance of an adequate airway.
EVALUATION AND MANAGEMENT
The goals in evaluating the potentially head-injured athlete are threefold: (1) the fact that a head injury has potentially occurred must be recognized; (2) the athletes requiring transport to a medical facility for further workup and treatment must be accurately identified; and (3) a decision must be made regarding when the athlete may return to competition. Obviously, detection of a potentially life-threatening or neurologically devastating injury is of paramount importance. In light of the possibility of second-impact syndrome, those athletes with mild head injuries must be cautiously observed and returned to play only when they are absolutely symptom free.
Any athlete who receives a blow to the head or any significant acceleration-deceleration-type force to the head should be presumed to have a possible head injury and should be thoroughly evaluated. The athlete should be evaluated for level of consciousness, steadiness of gait, orientation, and posttraumatic amnesia. Those players with grade 1 concussions generally should be observed for 20 to 30 minutes. If the sensorium totally clears, the neurologic examination is normal, and no residual symptoms are present, the athlete can usually be allowed to return to the game. Any persistent symptoms, such as headache, dizziness, or confusion, necessitate removal from competition and evaluation by a physician. An athlete with a grade 2 concussion should be removed from the game. Evaluation by a physician should be performed in most cases.17 In grade 3 concussion, the athlete's level of consciousness or orientation may be altered severely or for a prolonged time, or other neurologic deficits may be present. Transport to a hospital for emergency evaluation of the player by a neurosurgeon and diagnostic neuroimaging should be undertaken immediately if indicated. A cervical collar and long spine board should be used to prevent secondary injury to the player until spinal involvement can be definitely excluded. The unconscious athlete must be treated as any other unconscious patient. The airway must be firmly established and adequate breathing ensured. Airway protection may occasionally require emergent intubation. Hemodynamic monitoring is needed to ensure adequate circulation. Any focal neurologic changes, such as posturing or a dilated pupil, can be addressed with moderate hyperventilation.
The role of the certified athletic trainer during games and practices is to prevent injury and provide immediate first-aid care and triage. One of the more challenging aspects of game and practice coverage is the response to injuries involving the head and cervical spine. Knowledge concerning the clinical presentation and proper emergency care for these players with serious or catastrophic intracranial injuries is requisite for athletic trainers and medical personnel.
Before the season, the athletic training staff creates an emergency medical plan, instituting all procedures that must be followed during an emergency injury. The various entities making up the institutional medical team, the secondary support teams including local emergency medical services, ambulance units, and level 1 trauma centers (including helicopter transport), should be listed. Policies should go through the proper administrative protocols before being instituted. Also, all equipment to be used on a daily basis must be inspected and tested before each season, and in some instances, such equipment must be recalibrated annually.
The certified athletic trainer is trained in evaluation and screening skills and competencies in treating catastrophic head and spine injury. Initially, after a traumatic injury on the field, the athletic trainer performs an immediate basic life support evaluation to assess airway, breathing, and circulation. Then the secondary assessment, including neurologic and orthopaedic evaluation, takes place. In the conscious athlete, responses to questions allow for immediate information to be derived. The unconscious athlete creates a more complex and urgent evaluation.
The movement of an athlete from a prone or side-lying position requires teamwork among all members of the immediate and extended medical staff. The log-roll procedure remains the most accepted protocol in moving an athlete with a suspected head or cervical spine injury. The senior staff member should maintain the head position, while the other team members roll the athlete's head and body in unison to a supine position. The National Athletic Trainers' Association in conjunction with the Inter-Association Task Force for Appropriate Care of the Spine-Injured Athlete34 recently recommended that the facemask be removed, regardless of the athlete's current respiratory status, and that the helmet remain on the athlete unless it does not allow for immobilization of the head, the facemask cannot be safely removed, or the helmet interferes with immobilization during transport. The task force also recommended that the medical staff have sufficient practice in all aspects of emergency care before an incident.
Thus, it is important for caregivers at athletic contests to be cognizant of the spectrum of injuries that occur in response to both minor and major brain trauma. In addition to life-threatening hematomas, diffuse brain injury is common and can range from mild to severe. However, the former, as we are increasingly appreciating, may have more implications in terms of numbers of athletes affected, difficulty with diagnosis and classification, potential for long-term symptoms, and complexity with regard to return-to-play decisions.
REFERENCES
- 1.Strich SJ. Shearing of nerve fibers as a cause of brain damage due to head injury: a pathological study of twenty cases. Lancet. 1961;2:443–448. [Google Scholar]
- 2.Sweet RC, Miller JD, Lipper M, Kishore PR, Becker DP. Significance of bilateral abnormalities on the CT scan in patients with severe head injury. Neurosurgery. 1978;3:16–21. doi: 10.1227/00006123-197807000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Gennarelli TA. Cerebral concussion and diffuse brain injuries. In: Cooper PR, editor. Head Injury. 3rd ed. Baltimore, MD: Williams & Wilkins; 1993. pp. 137–158. [Google Scholar]
- 4.Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness: correlation of experimental and clinical observations on blunt head injuries. Brain. 1974;97:633–654. doi: 10.1093/brain/97.1.633. [DOI] [PubMed] [Google Scholar]
- 5.Pilz P. Axonal injury in head injury. Acta Neurochir Suppl (Wien) 1983;32:119–123. doi: 10.1007/978-3-7091-4147-2_17. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman RA, Bilaniuk LT, Gennarelli TA. Computed tomography of shearing injuries of the cerebral white matter. Radiology. 1978;127:393–396. doi: 10.1148/127.2.393. [DOI] [PubMed] [Google Scholar]
- 7.Levi L, Guilburd JN, Lemberger A, Soustiel JF, Feinsod M. Diffuse axonal injury: analysis of 100 patients with radiological signs. Neurosurgery. 1990;27:429–432. [PubMed] [Google Scholar]
- 8.Powell JW, Barber-Foss KD. Traumatic brain injury in high school athletes. JAMA. 1999;282:958–963. doi: 10.1001/jama.282.10.958. [DOI] [PubMed] [Google Scholar]
- 9.Schneider K, Zernicke RF. Computer simulation of head impact: estimation of head-injury risk during soccer heading. Int J Sport Biomech. 1988;4:358–371. [Google Scholar]
- 10.Gennarelli TA. Mechanisms of brain injury. J Emerg Med. 1993;11(suppl 1):5–11. [PubMed] [Google Scholar]
- 11.Leininger B, Gramling SE, Farrell AD, Kreutzer JS, Peck EA., 3d Neuropsychological deficits in symptomatic minor head injury patients after concussion and mild concussion. J Neurol Neurosurg Psychiatry. 1990;53:293–296. doi: 10.1136/jnnp.53.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutherford W. Post concussional symptoms: relationship to acute neurological indices, individual differences, and circumstances of injury. In: Levin H, Eisenberg H, Benton A, editors. Mild Head Injury. New York, NY: Oxford University Press; 1989. pp. 217–228. [Google Scholar]
- 13.Bergsneider M, Hovda DA, Shalmon E, et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- 14.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi H, Manaka S, Sano K. Changes in extracellular potassium concentration in cortex and brain stem during the acute phase of experimental closed head injury. J Neurosurg. 1981;55:708–717. doi: 10.3171/jns.1981.55.5.0708. [DOI] [PubMed] [Google Scholar]
- 16.Hovda DA, Becker DP, Katayama Y. Secondary injury and acidosis. J Neurotrauma. 1992;9(suppl 1):S47–S60. [PubMed] [Google Scholar]
- 17.Hovda DA, Prins M, Becker DP, Lee S, Bergsneider M, Martin NA. Neurobiology of concussion. In: Bailes JE, Lovell M, Maroon JC, editors. Sports-Related Concussion. St Louis, MO: Quality Medical Publishing; 1999. pp. 12–51. [Google Scholar]
- 18.Hubschmann OR, Kornhauser D. Effects of intraparenchymal hemorrhage on extracellular cortical potassium in experimental head trauma. J Neurosurg. 1983;59:289–293. doi: 10.3171/jns.1983.59.2.0289. [DOI] [PubMed] [Google Scholar]
- 19.Jamieson KG, Yelland JDN. Extradural hematoma: report of 167 cases. J Neurosurg. 1968;29:13–23. doi: 10.3171/jns.1968.29.1.0013. [DOI] [PubMed] [Google Scholar]
- 20.Servadei F. Prognostic factors in severely head injured adult patients with epidural haematomas. Acta Neurochir (Wien) 1997;139:273–278. doi: 10.1007/BF01808821. [DOI] [PubMed] [Google Scholar]
- 21.Bricolo A, Pasut LM. Extradural hematoma: toward zero mortality, a prospective study. Neurosurgery. 1984;14:8–12. doi: 10.1227/00006123-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Selig JM, Becker DP, Miller JD, Greenberg RP, Ward JD, Choi SC. Traumatic acute subdural hematoma: major mortality reduction in comatose patients treated within four hours. N Engl J Med. 1981;304:1511–1518. doi: 10.1056/NEJM198106183042503. [DOI] [PubMed] [Google Scholar]
- 23.Meek TD. Football injuries: acute subdural hematoma without loss of consciousness. Tex Med. 1970;66:58–59. [PubMed] [Google Scholar]
- 24.Kaste M, Waltimo O, Heiskanen O. Chronic bilateral subdural haematoma in adults. Acta Neurochir (Wien) 1979;48:231–236. doi: 10.1007/BF02056971. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, Suzuki J. Ultrastructural observations of the capsule of chronic subdural hematoma in various clinical stages. J Neurosurg. 1975;43:569–578. doi: 10.3171/jns.1975.43.5.0569. [DOI] [PubMed] [Google Scholar]
- 26.Robinson RG. Chronic subdural hematoma: surgical management in 133 patients. J Neurosurg. 1984;61:263–268. doi: 10.3171/jns.1984.61.2.0263. [DOI] [PubMed] [Google Scholar]
- 27.Schonauer M, Schisano G, Cimino R, Viola L. Space occupying contusions of cerebral lobes after closed brain injury: considerations about 51 cases. J Neurosurg Sci. 1979;23:279–288. [PubMed] [Google Scholar]
- 28.Centers for Disease Control. Sports-related recurrent brain injuries, United States. MMWR Morb Mortal Wkly Rep. 1997;46:224–227. [PubMed] [Google Scholar]
- 29.Cantu RC. Guidelines for return to contact sports after a cerebral concussion. Phys Sportsmed. 1986;14(10):75–83. doi: 10.1080/00913847.1986.11709197. [DOI] [PubMed] [Google Scholar]
- 30.Kelly JP, Rosenberg JH. Diagnosis and management of concussion in sports. Neurology. 1997;48:575–580. doi: 10.1212/wnl.48.3.575. [DOI] [PubMed] [Google Scholar]
- 31.Practice parameter: the management of concussion in sports (summary statement). Report of the Quality Standards Subcommittee. Neurology. 1997;48:581–585. doi: 10.1212/wnl.48.3.581. [DOI] [PubMed] [Google Scholar]
- 32.Report of the Sports Medicine Committee. Guidelines for the Management of Concussion in Sports. Denver, CO: Colorado Medical Society; 1991. May, [Google Scholar]
- 33.Kelly JP, Nichols JS, Filley CM, Lillenhei KO, Rubinstein D, Kleinschmidt-DeMasters BK. Concussion in sports: guidelines for the prevention of catastrophic outcome. JAMA. 1991;266:2867–2869. doi: 10.1001/jama.266.20.2867. [DOI] [PubMed] [Google Scholar]
- 34.Inter-Association Task Force for Appropriate Care of the Spine-Injured Athlete. Prehospital Care of the Spine-Injured Athlete. Dallas, TX: National Athletic Trainers' Association; 2001. [Google Scholar]