Abstract
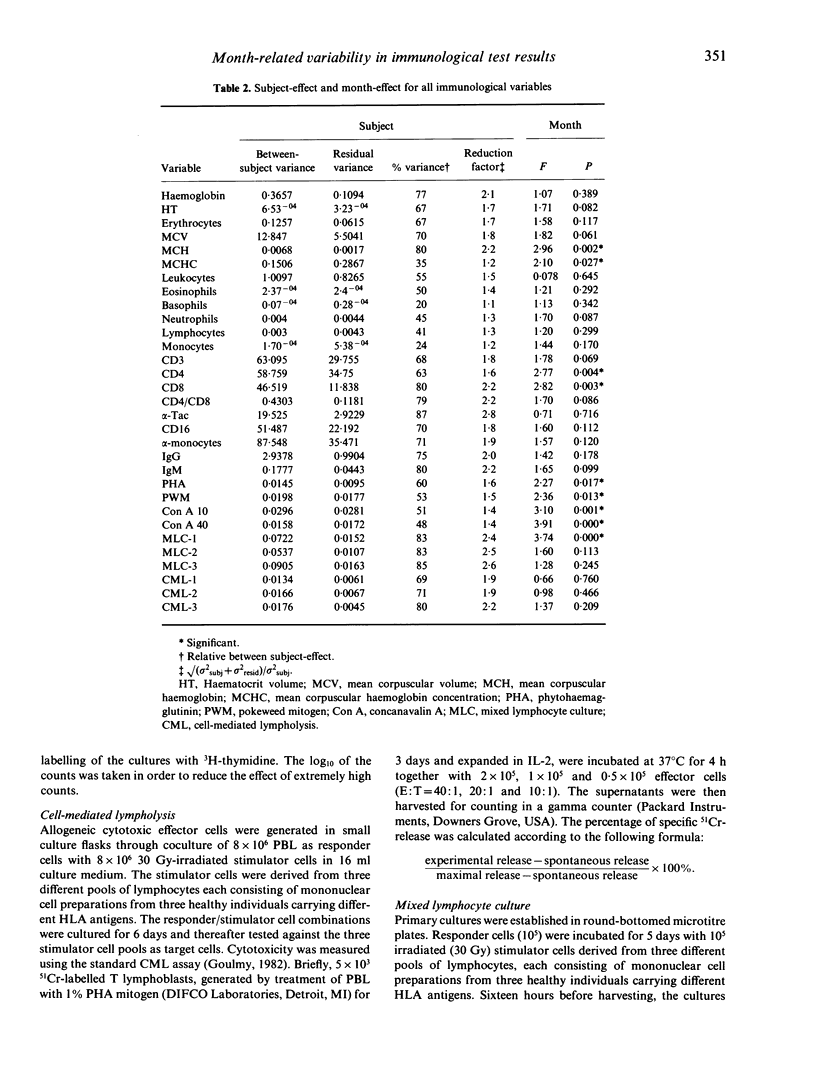
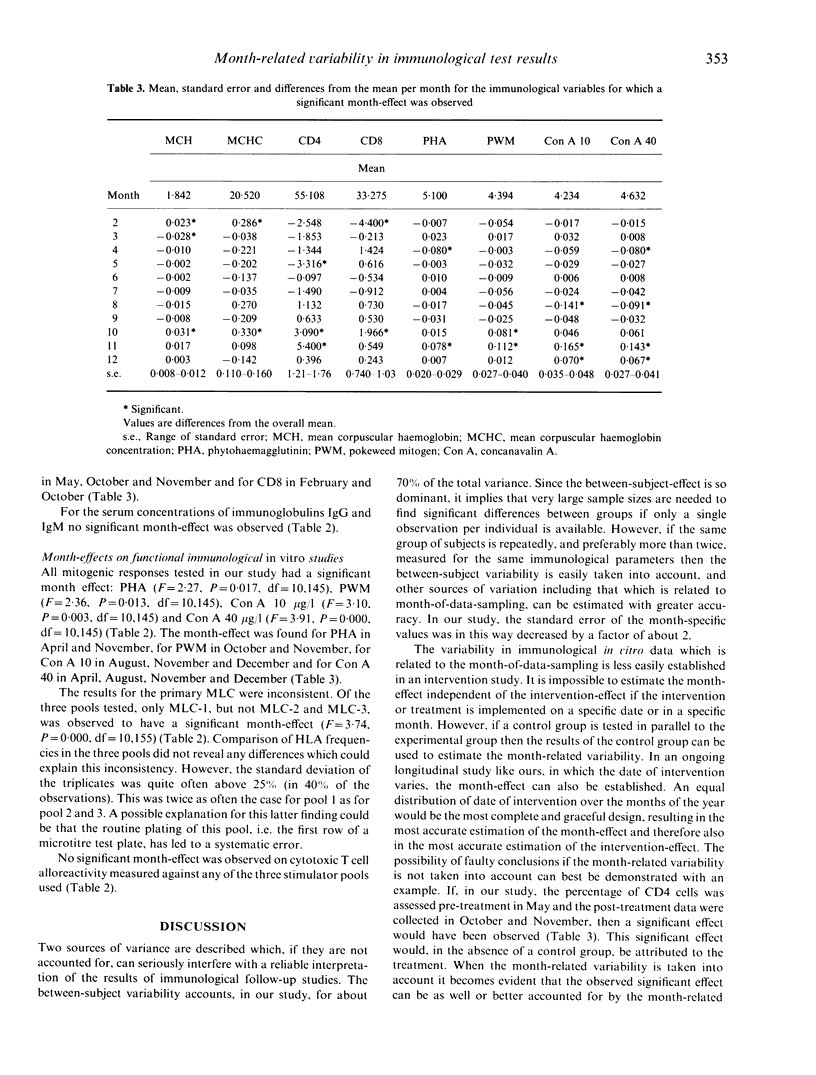
This longitudinal study was originally designed to detect changes in the in vitro immune response of healthy subjects as a result of a psychological intervention. In this study a significant proportion, about 70%, of the immunological variability in the test results was accounted for by the differences in immunological response levels of the subjects. Apart from this between-subject-effect, a significant proportion of the variability in test results was related to the month of data sampling. The month-effect was computed in such a way that the between-subject variation was taken into account. This resulted in a more accurate estimation of the month-effect. Even after correction for the intervention, i.e. the defence of the PhD thesis, the effect of month of data sampling remains significant for mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, percentage of CD4 and CD8 cells, and for the response to the mitogens phytohaemagglutinin, pokeweed mitogen and concanavalin A as well as the results for the mixed lymphocyte culture for one pool out of three. In contrast, no significant month-effect was observed for the whole blood cell counts, for the differential white blood cell counts as determined by monoclonal antibody staining for cell surface markers CD3, CD16, TAC and OKM1, nor for the immunoglobulin IgM and IgG serum levels. Likewise the cell-mediated lympholysis activities measured against three pools of stimulator cells remained unaltered. We discuss the implications for future immunological follow-up studies of the observation that a significant proportion of the variability in immunological test results is related to differences between subjects and to the month of data sampling.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boctor F. N., Charmy R. A., Cooper E. L. Seasonal differences in the rhythmicity of human male and female lymphocyte blastogenic responses. Immunol Invest. 1989 Jul;18(6):775–784. doi: 10.3109/08820138909030598. [DOI] [PubMed] [Google Scholar]
- Bratescu A., Teodorescu M. Circannual variations in the B cell/T cell ratio in normal human peripheral blood. J Allergy Clin Immunol. 1981 Oct;68(4):273–280. doi: 10.1016/0091-6749(81)90151-2. [DOI] [PubMed] [Google Scholar]
- Kaplan M. S., Byers V. S., Levin A. S., German D. F., Fudenberg H. H., Lecam L. N. Circadian rhythm of stimulated lymphocyte blastogenesis. A 24 hour cycle in the mixed leukocyte culture reaction and with SKSD stimulation. J Allergy Clin Immunol. 1976 Jul;58(1 Pt 2):180–189. doi: 10.1016/0091-6749(76)90153-6. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Glaser R. Methodological issues in behavioral immunology research with humans. Brain Behav Immun. 1988 Mar;2(1):67–78. doi: 10.1016/0889-1591(88)90007-4. [DOI] [PubMed] [Google Scholar]
- Lyngbye J., Kroll J. Quantitative immunoelectrophoresis of proteins in serum from a normal population: season-, age-, and sex-related variations. Clin Chem. 1971 Jun;17(6):495–500. [PubMed] [Google Scholar]
- MacMurray J. P., Barker J. P., Armstrong J. D., Bozzetti L. P., Kuhn I. N. Circannual changes in immune function. Life Sci. 1983 May 16;32(20):2363–2370. doi: 10.1016/0024-3205(83)90767-1. [DOI] [PubMed] [Google Scholar]
- Melnikov O. F., Nikolsky I. S., Dugovskaya L. A., Balitskaya N. A., Kravchuk G. P. Seasonal aspects of immunological reactivity of the human and animal organism. J Hyg Epidemiol Microbiol Immunol. 1987;31(2):225–230. [PubMed] [Google Scholar]
- Munch-Petersen B., Wallevik K., Faber M. Seasonal variations in UVR-induced DNA synthesis and in UVR inhibition of phytohaemagglutinin-stimulated proliferation of human lymphocytes. Scand J Clin Lab Invest. 1985 Feb;45(1):37–44. doi: 10.3109/00365518509160969. [DOI] [PubMed] [Google Scholar]
- Reinberg A., Schuller E., Delasnerie N., Clench J., Helary M. Rhthmes circadiens et circannuels des leucocytes, protéines totales, immunoglobulines A, G et M; étude chez 9 adultes jeunes et sains. Nouv Presse Med. 1977 Dec 3;6(41):3819–3823. [PubMed] [Google Scholar]
- Röcker L., Feddersen H. M., Hoffmeister H., Junge B. Jahreszeitliche Veränderungen diagnostisch wichtiger Blutbestandteile. Klin Wochenschr. 1980 Aug 1;58(15):769–778. doi: 10.1007/BF01478285. [DOI] [PubMed] [Google Scholar]
- Salden H. J., Bas B. M., Hermans I. T., Janson P. C. Analytical performance of three commercially available nephelometers compared for quantifying proteins in serum and cerebrospinal fluid. Clin Chem. 1988 Aug;34(8):1594–1596. [PubMed] [Google Scholar]
- Sternberg J. C. A rate nephelometer for measuring specific proteins by immunoprecipitin reactions. Clin Chem. 1977 Aug;23(8):1456–1464. [PubMed] [Google Scholar]
- Tavadia H. B., Fleming K. A., Hume P. D., Simpson H. W. Circadian rhythmicity of human plasma cortisol and PHA-induced lymphocyte transformation. Clin Exp Immunol. 1975 Oct;22(1):190–193. [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., Climie A., Muller H. K., Lugg D. J. Cell-mediated immunity in healthy adults in Antarctica and the sub-Antarctic. J Clin Lab Immunol. 1986 May;20(1):43–49. [PubMed] [Google Scholar]
- van Rood J. J., van Leeuwen A., Ploem J. S. Simultaneous detection of two cell populations by two-colour fluorescence and application to the recognition of B-cell determinants. Nature. 1976 Aug 26;262(5571):795–797. doi: 10.1038/262795a0. [DOI] [PubMed] [Google Scholar]



