Abstract
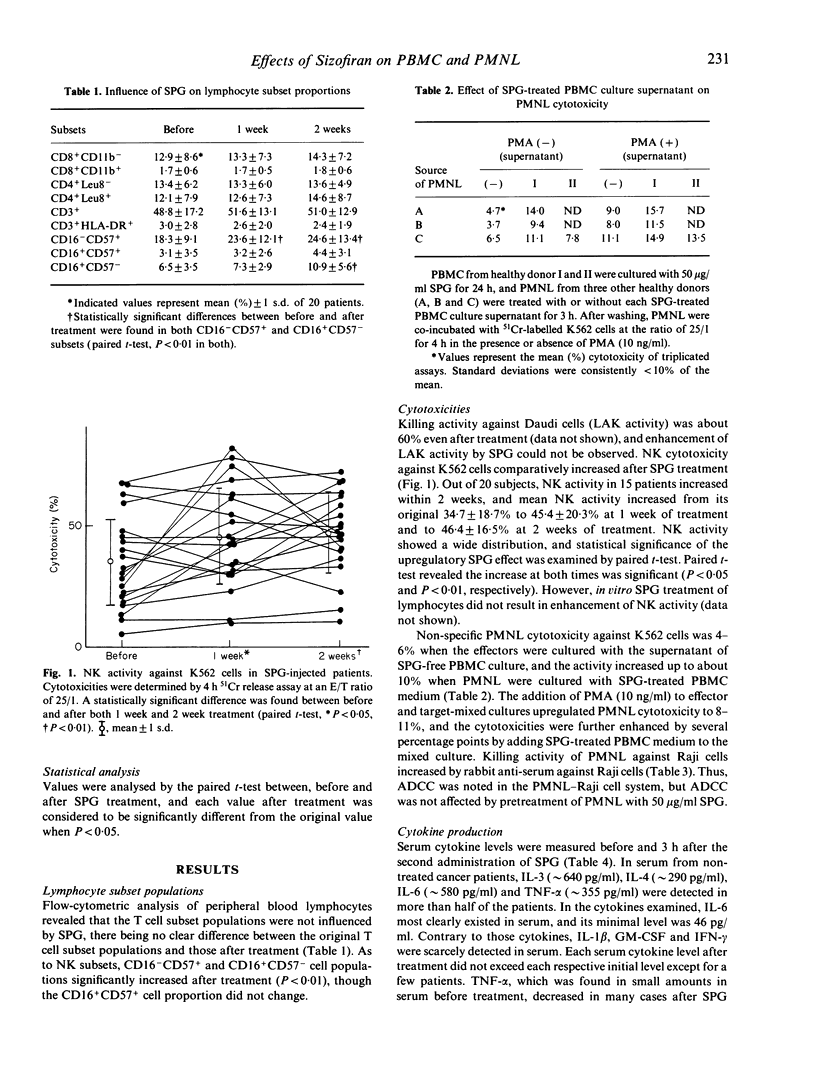
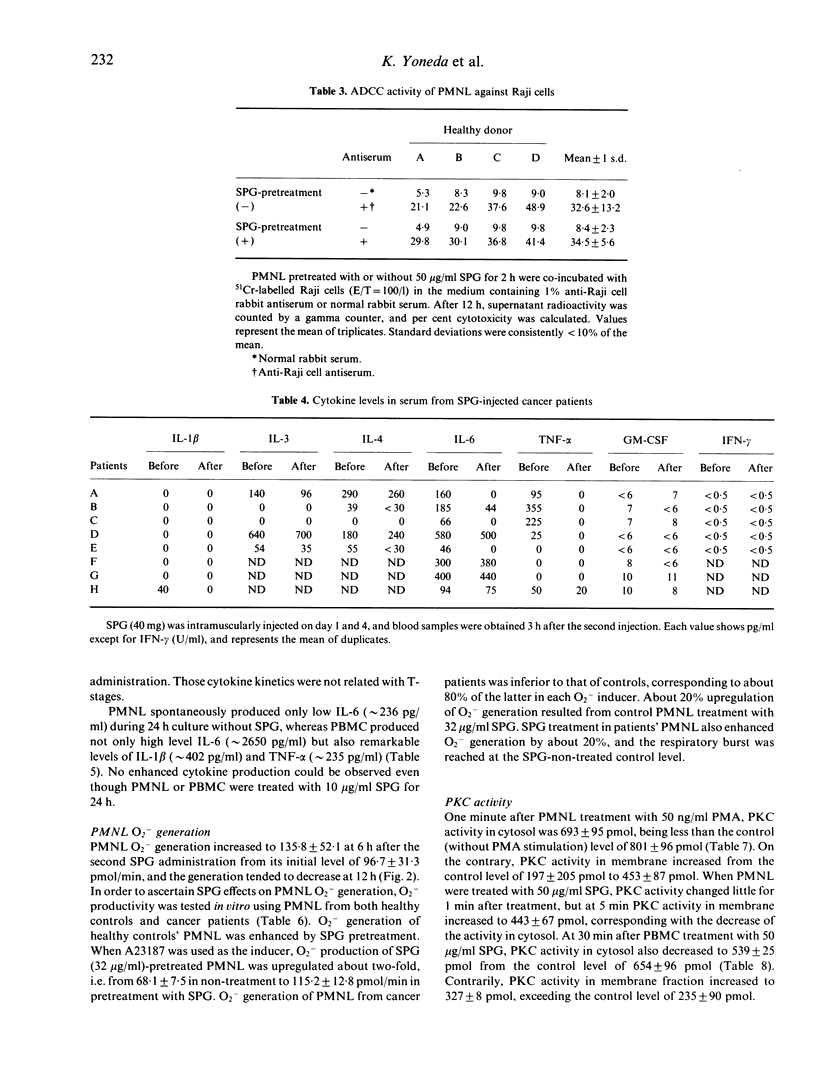
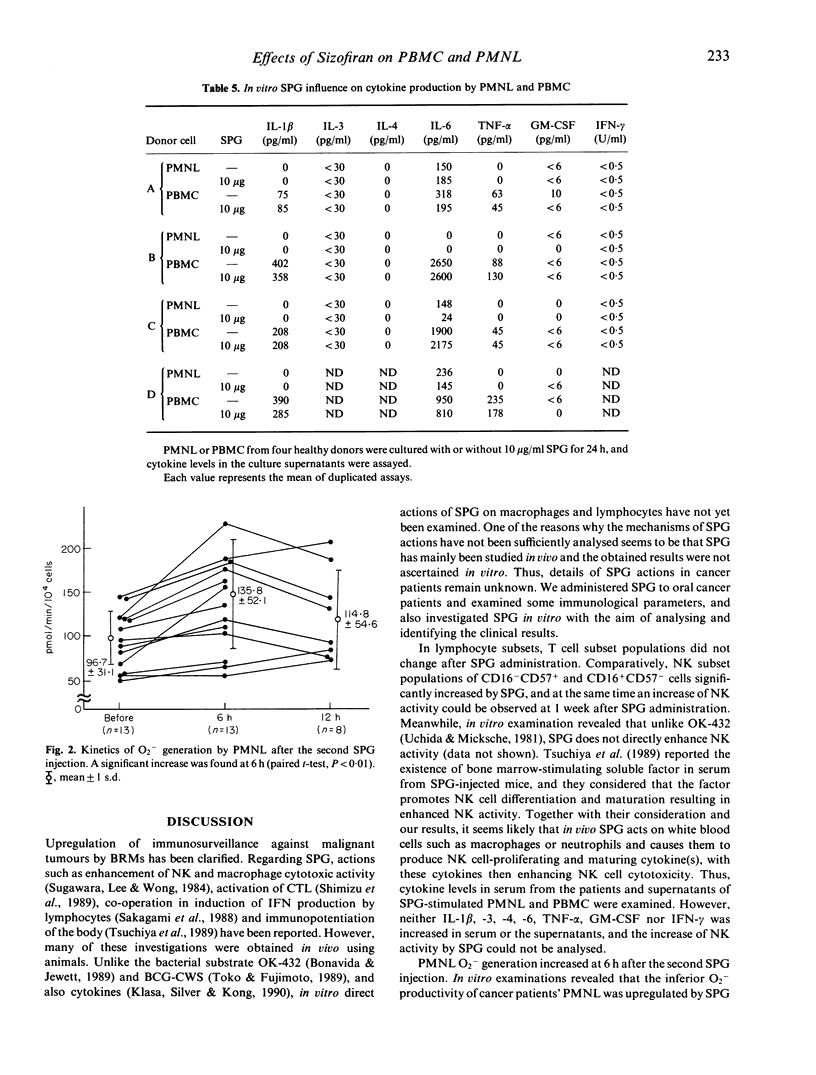
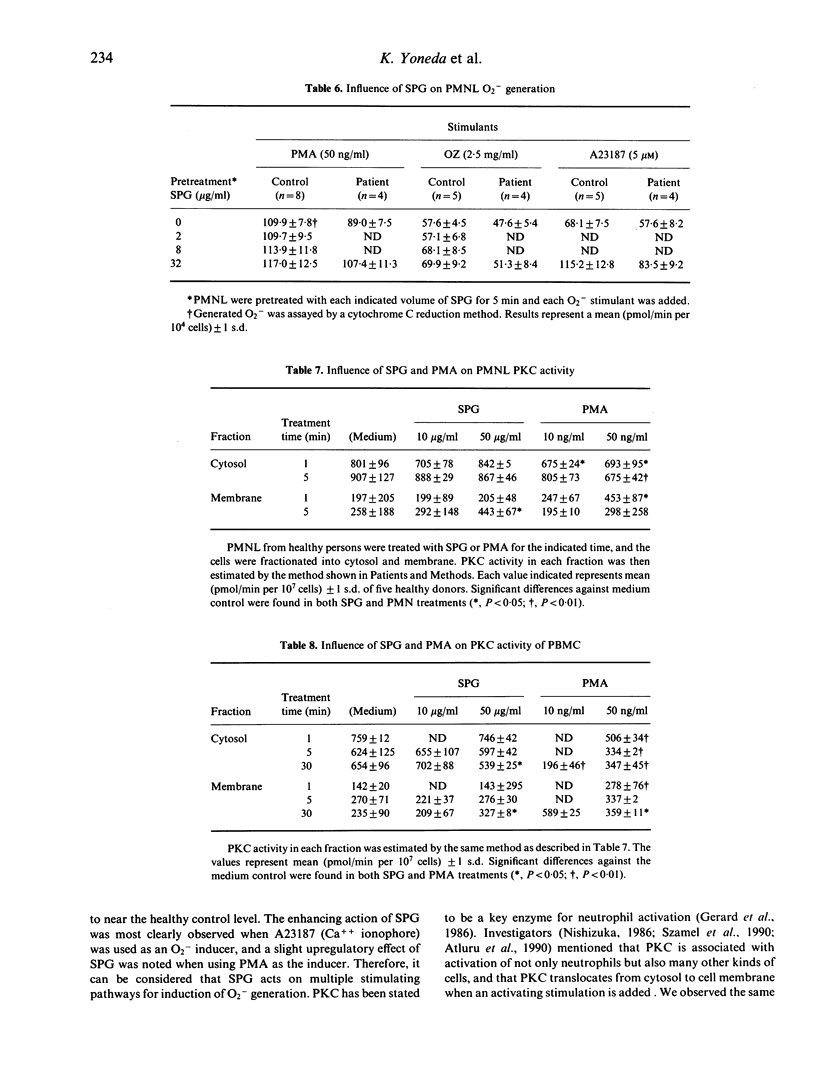
The effect of SPG on leukocytes has been studied in 20 patients with oral carcinoma and the actions have been analysed in vitro. SPG 1 mg/kg was administered intramuscularly twice weekly. Peripheral venous blood was collected before, and 1 week and 2 weeks after the initiation of SPG treatment. Both CD16+CD57- and CD16-CD57+ cell populations were significantly increased after treatment, but no T cell subset varied. While enhancement of lymphokine-activated killer activity could not be found, an increase in natural killer (NK) activity was observed in 15 of the subjects, and the mean NK level was significantly increased from an initial 34.7 +/- 18.7% to 46.4 +/- 16.5% after two weeks of injections. O2-production by polymorphonuclear leukocytes (PMNL) was stimulated 6 h after SPG injection. When PMNL were treated in vitro with SPG 32 micrograms/ml, enhanced O2-generation was induced and protein kinase C (PKC) activity in a membrane fraction increased. SPG did not directly affect non-specific PMNL killing of K562 cells or antibody-dependent cell mediated cytotoxicity against Raji cells, but non-specific PMNL killing was enhanced by culture-conditioned medium from peripheral blood mononuclear cells (PBMC) containing 10 micrograms/ml SPG. Interleukin-1 beta, -3, -4, -6, tumour necrosis factor-alpha, granulocyte-macrophage colony stimulating factor and IFN-gamma levels in the conditioned medium were not increased compared with medium from PBMC not treated with SPG. No clear increase of these cytokines was found in serum from the SPG-treated patients. From the above results, enhancement of PMNL O2-generation by SPG seems to be a direct action of SPG, but the mechanism of elevation of the non-specific killing activity of PMNL and NK cells is not known. Perhaps other cytokines than those assayed have participated in increasing non-specific cytotoxicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atluru D., Polam S., Atluru S., Woloschak G. E. Regulation of mitogen-stimulated human T-cell proliferation, interleukin-2 production, and interleukin-2 receptor expression by protein kinase C inhibitor, H-7. Cell Immunol. 1990 Sep;129(2):310–320. doi: 10.1016/0008-8749(90)90207-8. [DOI] [PubMed] [Google Scholar]
- Balazovich K. J., Smolen J. E., Boxer L. A. Endogenous inhibitor of protein kinase C: association with human peripheral blood neutrophils but not with specific granule-deficient neutrophils or cytoplasts. J Immunol. 1986 Sep 1;137(5):1665–1673. [PubMed] [Google Scholar]
- Bonavida B., Jewett A. Activation of human peripheral blood-derived monocytes by OK-432 (Streptococcus pyogenes): augmented cytotoxicity and secretion of TNF and synergy with rIFN-gamma. Cell Immunol. 1989 Oct 15;123(2):373–383. doi: 10.1016/0008-8749(89)90297-9. [DOI] [PubMed] [Google Scholar]
- Darrow T. L., Slingluff C. L., Seigler H. F. Autologous lymph node cell-derived tumor-specific cytotoxic T-cells for use in adoptive immunotherapy of human melanoma. Cancer. 1988 Jul 1;62(1):84–91. doi: 10.1002/1097-0142(19880701)62:1<84::aid-cncr2820620116>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Gerard C., McPhail L. C., Marfat A., Stimler-Gerard N. P., Bass D. A., McCall C. E. Role of protein kinases in stimulation of human polymorphonuclear leukocyte oxidative metabolism by various agonists. Differential effects of a novel protein kinase inhibitor. J Clin Invest. 1986 Jan;77(1):61–65. doi: 10.1172/JCI112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasa R. J., Silver H. K., Kong S. In vivo induction of lymphokine-activated killer cells by interleukin-2 splenic artery perfusion in advanced malignancy. Cancer Res. 1990 Aug 15;50(16):4906–4910. [PubMed] [Google Scholar]
- Makino R., Tanaka T., Iizuka T., Ishimura Y., Kanegasaki S. Stoichiometric conversion of oxygen to superoxide anion during the respiratory burst in neutrophils. Direct evidence by a new method for measurement of superoxide anion with diacetyldeuteroheme-substituted horseradish peroxidase. J Biol Chem. 1986 Sep 5;261(25):11444–11447. [PubMed] [Google Scholar]
- Morikawa K., Kamegaya S., Yamazaki M., Mizuno D. Hydrogen peroxide as a tumoricidal mediator of murine polymorphonuclear leukocytes induced by a linear beta-1,3-D-glucan and some other immunomodulators. Cancer Res. 1985 Aug;45(8):3482–3486. [PubMed] [Google Scholar]
- Nabi Z. F., Takeshige K., Hatae T., Minakami S. Hydrogen peroxide formation of polymorphonuclear leukocytes stimulated with cytochalasin D. Exp Cell Res. 1979 Dec;124(2):293–300. doi: 10.1016/0014-4827(79)90205-2. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Okamura K., Hamazaki Y., Yajima A., Noda K. Adjuvant immunotherapy: two randomized controlled studies of patients with cervical cancer. Biomed Pharmacother. 1989;43(3):177–181. doi: 10.1016/0753-3322(89)90212-6. [DOI] [PubMed] [Google Scholar]
- Sakagami Y., Mizoguchi Y., Shin T., Seki S., Kobayashi K., Morisawa S., Yamamoto S. Effects of an anti-tumor polysaccharide, schizophyllan, on interferon-gamma and interleukin 2 production by peripheral blood mononuclear cells. Biochem Biophys Res Commun. 1988 Sep 15;155(2):650–655. doi: 10.1016/s0006-291x(88)80544-8. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Chen J. T., Hirai Y., Nakayama K., Hamada T., Fujimoto I., Hasumi K., Masubuchi K. Augmentation of immune responses of pelvic lymph node lymphocytes in patients with cervical cancer by sizofiran. Nihon Sanka Fujinka Gakkai Zasshi. 1989 Dec;41(12):2013–2014. [PubMed] [Google Scholar]
- Sugawara I., Lee K. C., Wong M. Schizophyllan (SPG)-treated macrophages and anti-tumor activities against syngeneic and allogeneic tumor cells. I. Characteristics of SPG-treated macrophages. Cancer Immunol Immunother. 1984;16(3):137–144. doi: 10.1007/BF00205419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szamel M., Kracht M., Krebs B., Hübner U., Resch K. Activation signals in human lymphocytes: interleukin 2 synthesis and expression of high affinity interleukin 2 receptors require differential signalling for the activation of protein kinase C. Cell Immunol. 1990 Mar;126(1):117–128. doi: 10.1016/0008-8749(90)90305-b. [DOI] [PubMed] [Google Scholar]
- Tamura T., Sasaki Y., Shinkai T., Eguchi K., Sakurai M., Fujiwara Y., Nakagawa K., Minato K., Bungo M., Saijo N. Phase I study of combination therapy with interleukin 2 and beta-interferon in patients with advanced malignancy. Cancer Res. 1989 Feb 1;49(3):730–735. [PubMed] [Google Scholar]
- Toko T., Fujimoto S. Augmentation of anti-tumor immunity in low-responder mice by various biological response modifiers: analysis of effector mechanism. Jpn J Cancer Res. 1989 Dec;80(12):1212–1219. doi: 10.1111/j.1349-7006.1989.tb01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y., Igarashi M., Inoue M., Kumagai K. Cytokine-related immunomodulating activities of an anti-tumor glucan, sizofiran (SPG). J Pharmacobiodyn. 1989 Oct;12(10):616–625. doi: 10.1248/bpb1978.12.616. [DOI] [PubMed] [Google Scholar]
- Uchida A., Micksche M. In vitro augmentation of natural killing activity by OK-432. Int J Immunopharmacol. 1981;3(4):365–375. doi: 10.1016/0192-0561(81)90032-1. [DOI] [PubMed] [Google Scholar]


