Abstract
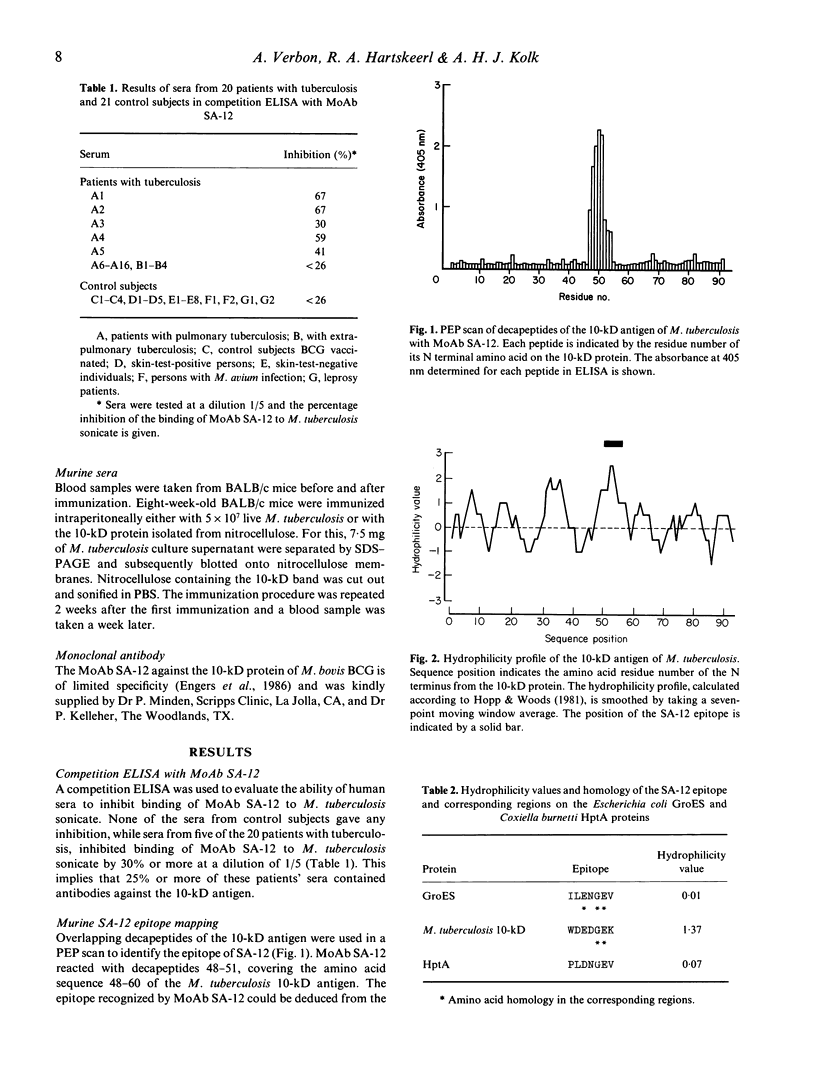
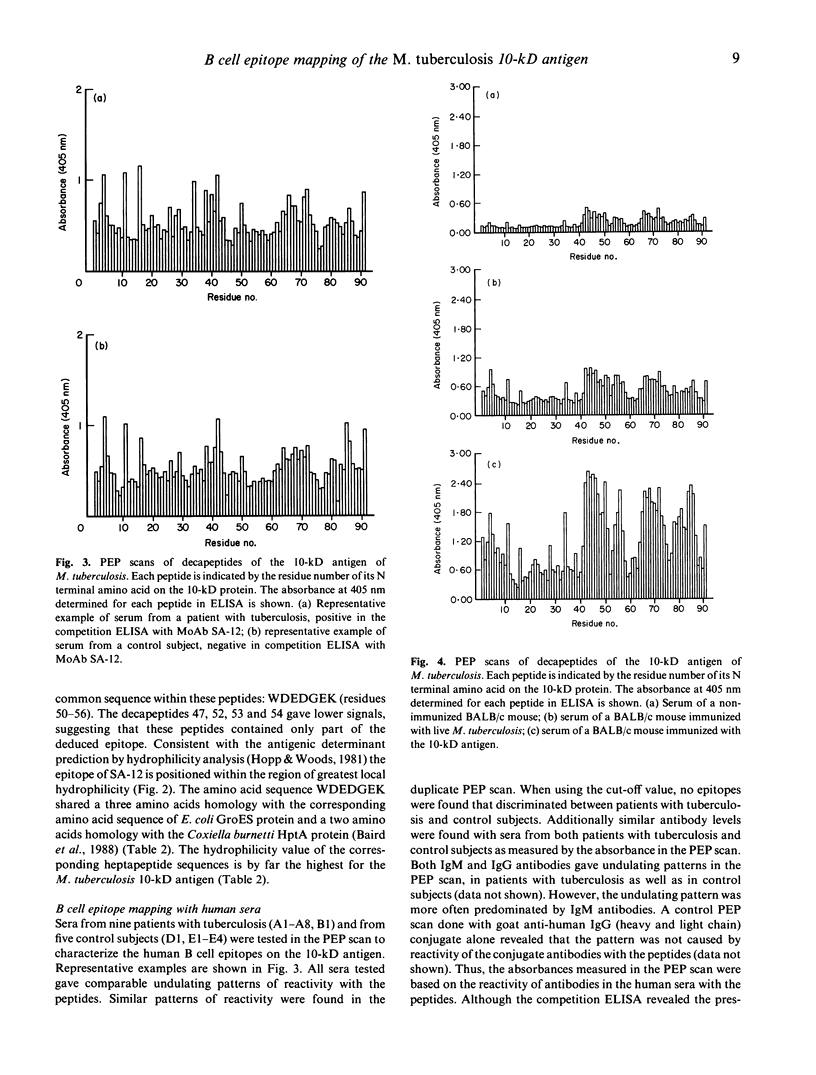
The human immune response to the 10-kD M. tuberculosis protein was studied by a competition ELISA using monoclonal antibody (MoAb) SA-12. Twenty-five per cent of the sera from 20 patients with tuberculosis and none from 21 control subjects inhibited binding of SA-12 to the 10-kD antigen. To characterize the antigenic parts of the 10-kD antigen, overlapping decapeptides according to the amino acid sequence of the 10-kD protein were synthesized. In total, 91 sequential decapeptides, with an overlap of nine amino acids, were tested in ELISA with MoAb SA-12, human and murine sera (PEP scan). SA-12 recognized the amino acid sequence WDEDGEK (amino acid 50-56). All human sera, from patients with tuberculosis as well as from control subjects, gave almost identical undulating patterns of reactivity with the decapeptides. No relationship was found between the ability of the patients' sera to inhibit binding of MoAb SA-12 and the binding of these sera to the decapeptides comprising the epitope recognized by SA-12 in the PEP scan. Apparently, antibodies in patients' sera against the 10-kD protein are predominantly directed against discontinuous epitopes and, consequently, the continuous epitopes as presented in the PEP scan are not suitable to discriminate between patients with tuberculosis and control subjects. In the PEP scan, sera from BALB/c mice, both non-immunized and immunized with either live M. tuberculosis or the 10-kD protein gave similar patterns of reactivity, albeit different from the patterns obtained with the human sera. However, after immunization of the mice, clearly increased levels of antibodies to primary structures of the 10-kD protein were observed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z. Antigenic structure of myoglobin: the complete immunochemical anatomy of a protein and conclusions relating to antigenic structures of proteins. Immunochemistry. 1975 May;12(5):423–438. doi: 10.1016/0019-2791(75)90010-5. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Guilbert B., Mahana W., Matsiota P., Ternynck T. Recognition of self and non-self constituents by polyspecific autoreceptors. Int Rev Immunol. 1988 Mar;3(1-2):1–15. doi: 10.3109/08830188809051179. [DOI] [PubMed] [Google Scholar]
- Baird P. N., Hall L. M., Coates A. R. A major antigen from Mycobacterium tuberculosis which is homologous to the heat shock proteins groES from E. coli and the htpA gene product of Coxiella burneti. Nucleic Acids Res. 1988 Sep 26;16(18):9047–9047. doi: 10.1093/nar/16.18.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird P. N., Hall L. M., Coates A. R. Cloning and sequence analysis of the 10 kDa antigen gene of Mycobacterium tuberculosis. J Gen Microbiol. 1989 Apr;135(4):931–939. doi: 10.1099/00221287-135-4-931. [DOI] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Intrinsic and extrinsic factors in protein antigenic structure. Science. 1985 Sep 6;229(4717):932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- Chan S. L., Reggiardo Z., Daniel T. M., Girling D. J., Mitchison D. A. Serodiagnosis of tuberculosis using an ELISA with antigen 5 and a hemagglutination assay with glycolipid antigens. Results in patients with newly diagnosed pulmonary tuberculosis ranging in extent of disease from minimal to extensive. Am Rev Respir Dis. 1990 Aug;142(2):385–389. doi: 10.1164/ajrccm/142.2.385. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Coates A. R., Nicolai H., Pallen M. J., Guy A., Chaparas S. D., Mitchison D. A. The 45 kilodalton molecule of Mycobacterium tuberculosis identified by immunoblotting and monoclonal antibodies as antigenic in patients with tuberculosis. Br J Exp Pathol. 1989 Apr;70(2):215–225. [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Guilbert B., Mahana W., Gilbert M., Mazie J. C., Avrameas S. Presence of natural autoantibodies in hyperimmunized mice. Immunology. 1985 Nov;56(3):401–408. [PMC free article] [PubMed] [Google Scholar]
- Hartskeerl R. A., van Rens R. M., Stabel L. F., de Wit M. Y., Klatser P. R. Selection and characterization of recombinant clones that produce Mycobacterium leprae antigens recognized by antibodies in sera from household contacts of leprosy patients. Infect Immun. 1990 Sep;58(9):2821–2827. doi: 10.1128/iai.58.9.2821-2827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J., Bothamley G. H., Jackett P. S. Immunodiagnostic assays for tuberculosis and leprosy. Br Med Bull. 1988 Jul;44(3):635–649. doi: 10.1093/oxfordjournals.bmb.a072273. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Bothamley G. H., Batra H. V., Mistry A., Young D. B., Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988 Nov;26(11):2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Sander C. On the use of sequence homologies to predict protein structure: identical pentapeptides can have completely different conformations. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1075–1078. doi: 10.1073/pnas.81.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatser P. R., De Wit M. Y., Kolk A. H., Hartskeerl R. A. Characterization of murine B-cell epitopes on the Mycobacterium leprae proline-rich antigen by use of synthetic peptides. Infect Immun. 1991 Jan;59(1):433–436. doi: 10.1128/iai.59.1.433-436.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk A. H., Evers R., Groothuis D. G., Gilis H., Kuijper S. Production and characterization of monoclonal antibodies against specific serotypes of Mycobacterium avium and the Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex. Infect Immun. 1989 Aug;57(8):2514–2521. doi: 10.1128/iai.57.8.2514-2521.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Meeker H. C., Williams D. L., Anderson D. C., Gillis T. P., Schuller-Levis G., Levis W. R. Analysis of human antibody epitopes on the 65-kilodalton protein of Mycobacterium leprae by using synthetic peptides. Infect Immun. 1989 Dec;57(12):3689–3694. doi: 10.1128/iai.57.12.3689-3694.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden P., Kelleher P. J., Freed J. H., Nielsen L. D., Brennan P. J., McPheron L., McClatchy J. K. Immunological evaluation of a component isolated from Mycobacterium bovis BCG with a monoclonal antibody to M. bovis BCG. Infect Immun. 1984 Nov;46(2):519–525. doi: 10.1128/iai.46.2.519-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Results of a World Health Organization-sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect Immun. 1986 Feb;51(2):718–720. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G. D. Prediction of chain turns in globular proteins on a hydrophobic basis. Nature. 1978 Apr 13;272(5654):586–590. doi: 10.1038/272586a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S. Polyvalent anti-DNA autoantibodies: immunochemical and biological significance. Int Rev Immunol. 1988 Mar;3(1-2):97–115. doi: 10.3109/08830188809051184. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Plikaytis B. B., Hyche A. D., Van Landingham R. M., Walker L. L. The Mycobacterium tuberculosis BCG-a protein has homology with the Escherichia coli GroES protein. Nucleic Acids Res. 1989 Feb 11;17(3):1254–1254. doi: 10.1093/nar/17.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Regenmortel M. H. Structural and functional approaches to the study of protein antigenicity. Immunol Today. 1989 Aug;10(8):266–272. doi: 10.1016/0167-5699(89)90140-0. [DOI] [PubMed] [Google Scholar]
- Verbon A., Kuijper S., Jansen H. M., Speelman P., Kolk A. H. Antigens in culture supernatant of Mycobacterium tuberculosis: epitopes defined by monoclonal and human antibodies. J Gen Microbiol. 1990 May;136(5):955–964. doi: 10.1099/00221287-136-5-955. [DOI] [PubMed] [Google Scholar]
- Wilkins E. G., Ivanyi J. Potential value of serology for diagnosis of extrapulmonary tuberculosis. Lancet. 1990 Sep 15;336(8716):641–644. doi: 10.1016/0140-6736(90)92144-7. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Haft D. H., Getzoff E. D., Tainer J. A., Lerner R. A., Brenner S. Identical short peptide sequences in unrelated proteins can have different conformations: a testing ground for theories of immune recognition. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5255–5259. doi: 10.1073/pnas.82.16.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R., Matsuo K., Yamazaki A., Nagai S., Terasaka K., Yamada T. Immunogenic protein MPB57 from Mycobacterium bovis BCG: molecular cloning, nucleotide sequence and expression. FEBS Lett. 1988 Nov 21;240(1-2):115–117. doi: 10.1016/0014-5793(88)80350-8. [DOI] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]


