Abstract
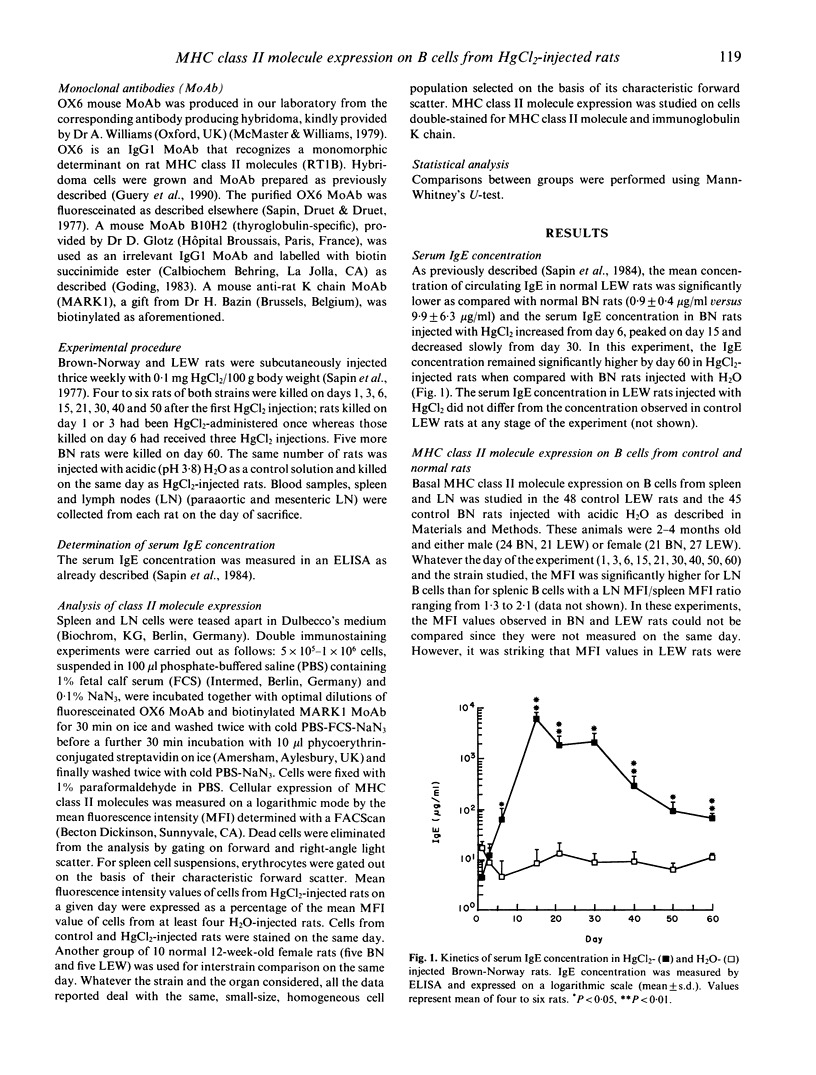
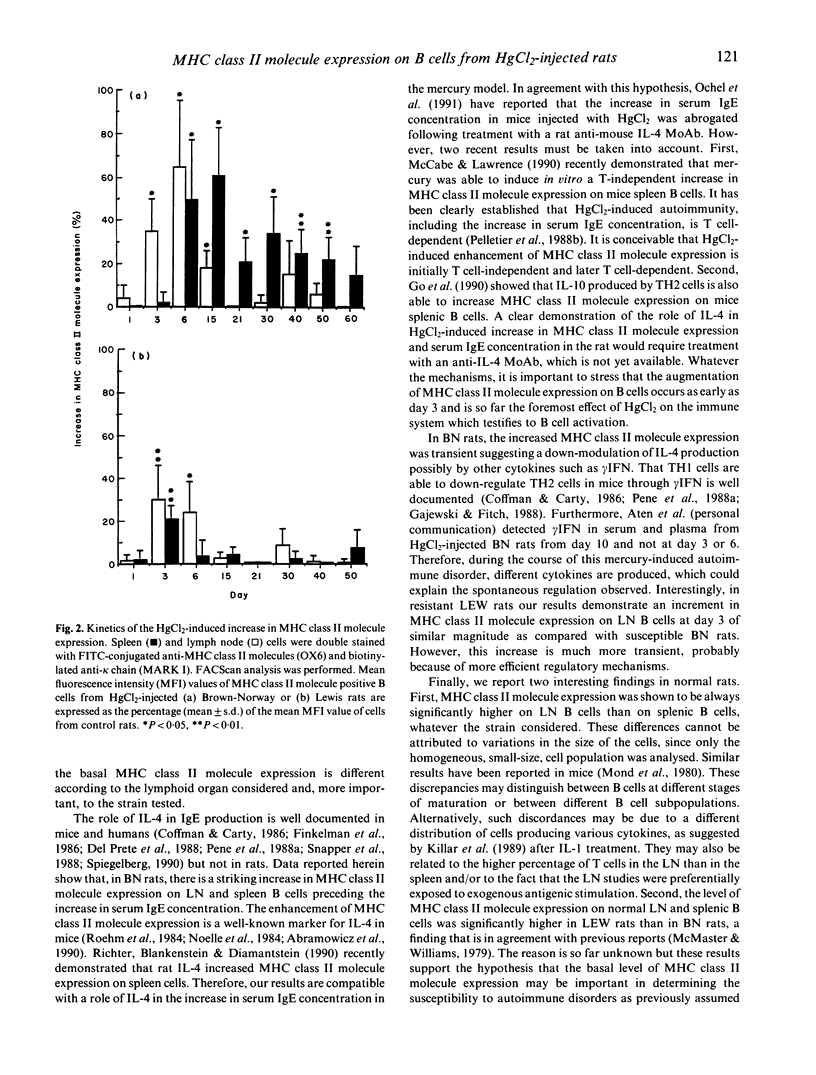
Administration of HgCl2 to the susceptible Brown-Norway (BN) rats induces an autoimmune disease characterized by a T-dependent polyclonal activation of B cells responsible for a dramatic increase in serum IgE concentration. The resistant Lewis (LEW) rats injected with HgCl2 do not exhibit such autoimmune manifestations. We show here that, upon HgCl2 injections, major histocompatibility complex (MHC) class II molecule expression is increased very early in lymph nodes and spleen B cells from both strains. So far, it is the earliest marker (day 3) of the effect of HgCl2 on the immune system. In both strains this enhancement is transient, but regulatory mechanisms are much more efficient in the resistant LEW strain than in the susceptible BN strain. In addition, we observed that MHC class II molecule expression on B cells differs according to the organ and the rat strain tested. All these findings are discussed in an attempt to underline the role of MHC class II molecule expression in the occurrence of mercury-induced autoimmunity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowicz D., Doutrelepont J. M., Lambert P., Van der Vorst P., Bruyns C., Goldman M. Increased expression of Ia antigens on B cells after neonatal induction of lymphoid chimerism in mice: role of interleukin 4. Eur J Immunol. 1990 Mar;20(3):469–476. doi: 10.1002/eji.1830200303. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Coffman R. L., Shrader B., Carty J., Mosmann T. R., Bond M. W. A mouse T cell product that preferentially enhances IgA production. I. Biologic characterization. J Immunol. 1987 Dec 1;139(11):3685–3690. [PubMed] [Google Scholar]
- Del Prete G., Maggi E., Parronchi P., Chrétien I., Tiri A., Macchia D., Ricci M., Banchereau J., De Vries J., Romagnani S. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988 Jun 15;140(12):4193–4198. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Snapper C. M., Ohara J., Paul W. E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Go N. F., Castle B. E., Barrett R., Kastelein R., Dang W., Mosmann T. R., Moore K. W., Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990 Dec 1;172(6):1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M., Druet P., Gleichmann E. TH2 cells in systemic autoimmunity: insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991 Jul;12(7):223–227. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- Guéry J. C., Druet E., Glotz D., Hirsch F., Mandet C., De Heer E., Druet P. Specificity and cross-reactive idiotypes of anti-glomerular basement membrane autoantibodies in HgCl2-induced autoimmune glomerulonephritis. Eur J Immunol. 1990 Jan;20(1):93–100. doi: 10.1002/eji.1830200114. [DOI] [PubMed] [Google Scholar]
- Hirsch F., Couderc J., Sapin C., Fournie G., Druet P. Polyclonal effect of HgCl2 in the rat, its possible role in an experimental autoimmune disease. Eur J Immunol. 1982 Jul;12(7):620–625. doi: 10.1002/eji.1830120716. [DOI] [PubMed] [Google Scholar]
- Killar L. M., Hatfield C. A., Carding S. R., Pan M., Winterrowd G. E., Bottomly K. In vivo administration of interleukin 1 elicits increased Ia antigen expression on B cells through the induction of interleukin 4. Eur J Immunol. 1989 Dec;19(12):2205–2210. doi: 10.1002/eji.1830191205. [DOI] [PubMed] [Google Scholar]
- McCabe M. J., Jr, Lawrence D. A. The heavy metal lead exhibits B cell-stimulatory factor activity by enhancing B cell Ia expression and differentiation. J Immunol. 1990 Jul 15;145(2):671–677. [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Mond J. J., Carman J., Sarma C., Ohara J., Finkelman F. D. Interferon-gamma suppresses B cell stimulation factor (BSF-1) induction of class II MHC determinants on B cells. J Immunol. 1986 Dec 1;137(11):3534–3537. [PubMed] [Google Scholar]
- Mond J. J., Kessler S., Finkelman F. D., Paul W. E., Scher I. Heterogeneity of Ia expression on normal B cells, neonatal B cells, and on cells from B cell-defective CBA/N mice. J Immunol. 1980 Apr;124(4):1675–1682. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Noelle R., Krammer P. H., Ohara J., Uhr J. W., Vitetta E. S. Increased expression of Ia antigens on resting B cells: an additional role for B-cell growth factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6149–6153. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochel M., Vohr H. W., Pfeiffer C., Gleichmann E. IL-4 is required for the IgE and IgG1 increase and IgG1 autoantibody formation in mice treated with mercuric chloride. J Immunol. 1991 May 1;146(9):3006–3011. [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Guettier C., Vial M. C., Mandet C., Nochy D., Bazin H., Druet P. HgC12 induces T and B cells to proliferate and differentiate in BN rats. Clin Exp Immunol. 1988 Feb;71(2):336–342. [PMC free article] [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Rossert J., Vial M. C., Mandet C., Druet P. Autoreactive T cells in mercury-induced autoimmunity. Ability to induce the autoimmune disease. J Immunol. 1988 Feb 1;140(3):750–754. [PubMed] [Google Scholar]
- Powrie F., Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990 Dec 1;172(6):1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Paliard X., Banchereau J., Spits H., De Vries J. E. IgE production by normal human B cells induced by alloreactive T cell clones is mediated by IL-4 and suppressed by IFN-gamma. J Immunol. 1988 Aug 15;141(4):1218–1224. [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Wideman J., Bonnefoy J. Y., De Vries J. E. Interleukin 5 enhances interleukin 4-induced IgE production by normal human B cells. The role of soluble CD23 antigen. Eur J Immunol. 1988 Jun;18(6):929–935. doi: 10.1002/eji.1830180615. [DOI] [PubMed] [Google Scholar]
- Richter G., Blankenstein T., Diamantstein T. Evolutionary aspects, structure, and expression of the rat interleukin 4 gene. Cytokine. 1990 May;2(3):221–228. doi: 10.1016/1043-4666(90)90020-t. [DOI] [PubMed] [Google Scholar]
- Roehm N. W., Leibson H. J., Zlotnik A., Kappler J., Marrack P., Cambier J. C. Interleukin-induced increase in Ia expression by normal mouse B cells. J Exp Med. 1984 Sep 1;160(3):679–694. doi: 10.1084/jem.160.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapin C., Druet E., Druet P. Induction of anti-glomerular basement membrane antibodies in the Brown-Norway rat by mercuric chloride. Clin Exp Immunol. 1977 Apr;28(1):173–179. [PMC free article] [PubMed] [Google Scholar]
- Sapin C., Hirsch F., Delaporte J. P., Bazin H., Druet P. Polyclonal IgE increase after HgCl2 injections in BN and LEW rats: a genetic analysis. Immunogenetics. 1984;20(3):227–236. doi: 10.1007/BF00364205. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Finkelman F. D., Paul W. E. Regulation of IgG1 and IgE production by interleukin 4. Immunol Rev. 1988 Feb;102:51–75. doi: 10.1111/j.1600-065x.1988.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Spickett G. P., Brandon M. R., Mason D. W., Williams A. F., Woollett G. R. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983 Sep 1;158(3):795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L. The role of interleukin-4 in IgE and IgG subclass formation. Springer Semin Immunopathol. 1990;12(4):365–383. doi: 10.1007/BF00225324. [DOI] [PubMed] [Google Scholar]
- Yokota T., Coffman R. L., Hagiwara H., Rennick D. M., Takebe Y., Yokota K., Gemmell L., Shrader B., Yang G., Meyerson P. Isolation and characterization of lymphokine cDNA clones encoding mouse and human IgA-enhancing factor and eosinophil colony-stimulating factor activities: relationship to interleukin 5. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7388–7392. doi: 10.1073/pnas.84.21.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]


