Abstract
Objective:
Postural stability and neuropsychological testing are gradually becoming integral parts of postconcussion assessment in athletes. Clinicians, however, sometimes question the viability of instituting preseason baseline testing and the value of these results in making return-to-play decisions. Our purpose was to examine the course of recovery on various postural stability and neuropsychological measures after sport-related concussion. A secondary goal was to determine if loss of consciousness and amnesia, both of which are heavily weighted in most of the concussion classification systems, affect the rate of recovery.
Design and Setting:
All subjects underwent a battery of baseline postural stability and neuropsychological tests before the start of their respective seasons. Any athletes subsequently injured were followed up at postinjury days 1, 3, and 5. Matched control subjects were assessed using the same test battery at the same time intervals.
Subjects:
We studied 36 Division I collegiate athletes who sustained a concussion and 36 matched control subjects.
Measurements:
We assessed postural stability using the Sensory Organization Test on the NeuroCom Smart Balance Master System and the Balance Error Scoring System. Neurocognitive functioning was measured with several neuropsychological tests: Trail-Making Test, Wechsler Digit Span Test, Stroop Color Word Test, and Hopkins Verbal Learning Test.
Results:
Injured subjects demonstrated postural stability deficits, as measured on both the Sensory Organization Test and Balance Error Scoring System. These deficits were significantly worse than both preseason scores and matched control subjects' scores on postinjury day 1. Only the results on the Trail-Making Test B and Wechsler Digit Span Test Backward resulted in a logical recovery curve that could explain lowered neuropsychological performance due to concussive injury. Significant differences were revealed between the control and injured groups at day 1 postinjury, but a significant decline between baseline and postinjury scores was not demonstrated. Loss of consciousness and amnesia were not associated with increased deficits or slowed recovery on measures of postural stability or neurocognitive functioning.
Conclusions:
Athletes with cerebral concussion demonstrated acute balance deficits, which are likely the result of not using information from the vestibular and visual systems effectively. Neurocognitive deficits are more difficult to identify in the acute stages of concussion, although concentration, working memory, immediate memory recall, and rapid visual processing appear to be mildly affected. More research is necessary to determine the best neuropsychological test battery for assessing sport-related concussion.
Keywords: mild head injury, balance, neurocognitive function
The use of neuropsychological and postural stability testing for the management of sport-related cerebral concussion is gradually becoming more commonplace among sports medicine clinicians. Recent research suggests that the use of a comprehensive approach may assist the athletic trainer and team physician in identifying signs of a concussion not easily detected during a routine clinical examination.1–3 Similarly, the use of these tests can eliminate some of the guesswork from the return-to-play (RTP) decision after concussion, as the subjective nature of symptoms associated with the injury make this assessment uniquely challenging. Despite the potentially catastrophic consequences of an athlete's premature return to competition after concussion, RTP decisions are often based on speculation rather than certainty. The life-threatening consequences of second-impact syndrome are well documented in the literature4–8 and should be a legitimate concern for all sports medicine personnel.
Awareness is clearly increased regarding the dangers of sport-related concussion compared with 10 years ago. Although football is generally recognized as the sport most often associated with concussion, moderate to high incidences of concussion have been noted in basketball, softball, soccer, baseball, boxing, rugby, and ice hockey.9–11 Despite rule changes and equipment modifications aimed at reducing concussion in sport, these injuries still occur frequently. The incidence of recurrent injury (15% to 20%) is higher than that of the initial injury (5% to 10%) in most sports,12–15 thereby suggesting the need to validate objective assessment techniques that enable the clinician to grade the initial injury and minimize the risk of a second or third such injury. Reports of the cumulative effects of multiple head injuries, as well as multiple head impacts, on long-term cognitive functioning are causing clinicians to rethink their approach to managing concussion in sport.16–20 Athletes sustaining concussion have displayed deficiencies in neurocognitive functioning such as attention, memory, concentration, and information processing as a result of cerebral concussion.16,21–31 Additionally, the areas of the brain disrupted as a result of concussion or traumatic brain injury have been reported to be responsible for the maintenance of postural equilibrium.1–3,32–41 As a result of these findings, neurocognitive and postural stability measures have been proposed as means by which concussion can be objectively assessed. Traditionally, clinicians have used the Romberg test for assessing disequilibrium in head-injured athletes, but only recently has computerized posturography been available to offer a more objective, challenging, and quantifiable assessment.
Although published reports have contributed significantly to our understanding of injury mechanisms, high-risk sports and positions, and symptoms associated with concussion, they have been limited in their ability to help us substantiate the recommended concussion grading scales and RTP guidelines. Most experts would agree that the nearly 20 proposed grading scales and RTP guidelines are very safe when adhered to closely; however, clinicians often question their practicality. While these scales and guidelines may be safe, most are based on a collection of clinical observations rather than experimentally based research findings. Much of the disagreement surrounding these grading scales and RTP guidelines stems from a dearth of scientific data to support them, which has resulted in the lack of a gold standard in the management of sport-related concussion. Most of the grading scales place significant weight on loss of consciousness (LOC) and amnesia, yet these have not proven to be predictive of neurocognitive decline, motor insufficiency, or long-term disability.
Our purpose was to investigate the effect of concussion on postural stability and neurocognitive function in athletes and furthermore to determine if injured players experiencing LOC and amnesia had a slower recovery than those who did not experience LOC or amnesia. The findings may provide insight into a more comprehensive approach for obtaining objective information with which clinicians can assess sport-related concussion.
METHODS
Subjects consisted of 36 collegiate athletes who sustained a concussion during either practice or competition and 36 recruited matched control subjects. Injured players who had received preseason baseline neuropsychological and postural stability testing were assessed on days 1, 3, and 5 postinjury. The matched control subjects were athletes of the same approximate age, height, and weight as their injured counterparts who had played approximately the same amount of time on the day of their matched counterparts' injuries. They were assessed according to the same schedule as the injured subjects. Control subjects who had sustained a concussion within 6 months of testing or who presented with a vestibular deficit or an acute musculoskeletal injury that affected postural equilibrium were excluded from the study.
Concussion was defined as injury to the brain caused by a sudden acceleration or deceleration of the head that resulted in any immediate, but temporary, alteration in brain functions, such as loss of consciousness, blurred vision, dizziness, amnesia, or memory impairment. All injured athletes were referred to the Sports Medicine Research Laboratory after being evaluated by a certified athletic trainer and team physician. All subjects were informed of the procedures and inherent risks of the investigation. They read and signed an informed consent form in accordance with the University of North Carolina's Academic Affairs Institutional Review Board, which approved the study. In addition to the postural stability and cognitive assessments, any current signs and symptoms associated with concussion were recorded at the time of the assessment.
Postural Stability Assessment
We took 2 measures of postural stability during each assessment. The first measure was the Sensory Organization Test (SOT) administered on the NeuroCom Smart Balance Master System (NeuroCom International, Inc, Clackamas, OR) (Figure 1). This forceplate system measures vertical ground reaction forces produced by the body's center of gravity moving around a fixed base of support. The SOT is designed to systematically disrupt the sensory selection process by altering available somatosensory or visual information or both while measuring a subject's ability to minimize postural sway.
Figure 1.
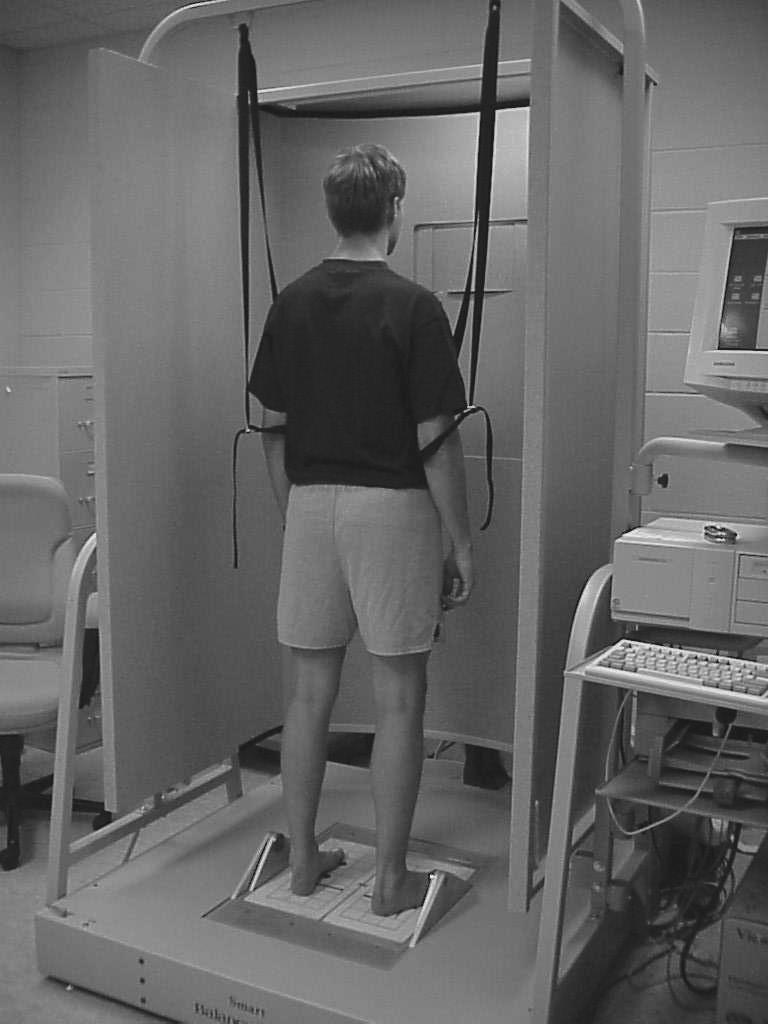
Smart Balance Master System.
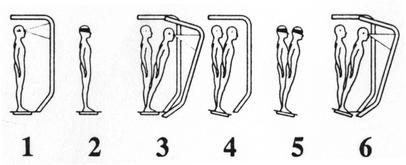
The test protocol consists of 18 total trials (20 seconds each), in which the subject is asked to stand as motionless as possible with the feet shoulder-width apart. Three trials are completed for each of the 6 conditions presented in Figure 2, in which 3 different visual conditions (eyes open, eyes closed, sway-referenced visual surround) are crossed with 2 different surface conditions (fixed, sway referenced). The term sway referencing involves the tilting of the support surface or visual surround (or both) to directly follow the athlete's center-of-gravity (COG) sway. During sway-referenced support-surface conditions (4–6), the forceplate tilts synchronously with the subject's anterior-posterior (A-P) COG sway. Similarly, during sway-referenced visual-surround conditions (3, 6), the visual surround tilts synchronously with A-P COG sway. Sway referencing causes orientation of the support surface or surround to remain constant relative to body position. The SOT can assess the subject's ability to ignore the inaccurate information from the sway-referenced sense(s). A composite equilibrium score describing a person's overall level of performance during all of the trials in the SOT is calculated, with higher scores indicating better balance performance. The composite score is a weighted average of the equilibrium scores from the 18 trials (3 for each of the 6 conditions); the scores from conditions 1 and 2 are weighted slightly less than those of conditions 3 through 6. The equilibrium scores from each of the trials represent a nondimensional percentage comparing the subject's peak amplitude of A-P sway with the theoretical A-P limit of stability. The theoretical limit of stability is based on the individual's height and size of the base of support. It represents an angle at which the person can lean in any direction before the COG would move beyond a point that allows him or her to remain upright (ie, point of falling). Lower percentages result in a higher (better) composite score. As part of the SOT, relative differences between the equilibrium scores of various conditions are calculated using ratios to reveal specific information about each of the sensory modalities involved with maintaining balance. These ratios are useful in identifying sensory integration problems, as lower ratios indicate an inability to compensate for disruptions in selected sensory inputs. The vestibular ratio is computed by using scores obtained in condition 5 (eyes closed, sway-referenced platform) and condition 1 (eyes open, fixed platform). This ratio indicates the relative reduction in postural stability when visual and somatosensory inputs are simultaneously disrupted. The visual ratio is obtained by comparing condition 4 with condition 1, and the somatosensory ratio compares condition 2 with condition 1.
Figure 2.
Six testing conditions for Sensory Organization Test used with NeuroCom's Smart Balance Master System.
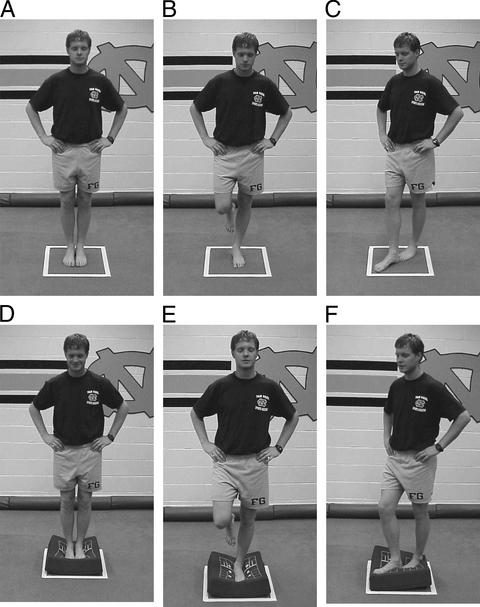
The second test of postural stability was the Balance Error Scoring System (BESS) and served as a clinical evaluation measure independent of the forceplate measure. Three different stances (double, single, and tandem) were completed twice, once while on a firm surface and once while on a 10-cm-thick piece of medium-density foam (thickness 45 cm2 × 13 cm, density 60 kg/m3, load deflection 80–90 kg) for a total of 6 20-second trials. Subjects were instructed to assume the required stance by placing their hands on their iliac crests and were informed that when they closed their eyes, the test would begin. During the single-limb stance trials, subjects were asked to maintain the contralateral limb in 20° of hip flexion and 45° of knee flexion. Additionally, we asked subjects to stand quietly and as motionless as possible in the stance position, keeping their hands on their iliac crests and their eyes closed (Figure 3). We demonstrated the 6 types of errors (Table 1) before testing and instructed the subjects to minimize the number of errors during the test trials. They were further told to make any necessary adjustments in the event that they lost their balance and to return to the testing position as quickly as possible. Performance was scored by adding 1 error point for each error committed. This method of testing has been previously described in detail and has been shown to be both valid and reliable using normal subjects.42
Figure 3.
Balance Error Scoring System (BESS) performed on the firm surface (top, A–C) and foam surface (bottom, D–F).
Table 1.
Balance Error Scoring System

Neurocognitive Assessment
We assessed neurocognitive function using standardized administration and scoring procedures in a quiet, controlled environment. The tests were administered by certified athletic trainers, all of whom had been previously trained in administration of the tests. A battery of 5 neuropsychological tests was used to assess various aspects of cognitive function often depressed after concussion. The test descriptions follow.
Trail-Making Test A
(Reitan Neuropsychological Laboratory, Tucson, AZ). Subjects completing this test are instructed to sequentially trace a list of 25 numbers on a piece of paper as fast as possible using a pen. This is an attentional and visual tracking task requiring rapid visual processing. The time required for successful completion is recorded, adding 1 second for each sequential error committed. Adding of 1 second for each sequential error is a modification to the standard administration.
Trail-Making Test B
(Reitan Neuropsychological Laboratory, Tucson, AZ). Similar to the Trail-Making Test A, subjects are instructed to connect circles containing both numbers (1–13) and alphabet letters (A–L) in alternating numeric and alphabetic fashion as fast as possible using a pen. This task assesses working memory and rapid visual processing.
Wechsler Digit Span Test (WDST)
(Psychological Corporation, San Antonio, TX). The WDST consists of a 2-part protocol and is used to examine a patient's concentration and immediate memory recall. During both parts of the test, subjects are presented with a series of numbers and asked to repeat the digits in either the same order (Digits Forward) for the first part or in the reverse order (Digits Backward) for the second part.
Stroop Color Word Test
(Stoelting Co, Wood Dale, IL). The Stroop Color Word Test is designed to assess cognitive flexibility, attention, and response inhibition by examining a subject's ability to separate word and color-naming stimuli through the use of 3 separate subtests. Only subtest 3 was analyzed in this study. The words RED, BLUE, and GREEN are randomly listed in 5 columns of 20 items. Subjects are instructed to read aloud the color of the print for each item (possibly the word RED printed in green ink). Subjects are given 45 seconds to complete as many items as possible.
Hopkins Verbal Learning Test (HVLT)
(Johns Hopkins University, Baltimore, MD). Each form of the HVLT consists of a 12-item word list composed of 4 words from 3 semantic categories used for assessing verbal memory and learning. The subject is instructed to listen carefully and memorize the word list. The subject then recalls as many words as possible in any order. The examiner records the number of correct responses, and the same procedure is repeated for 2 more trials. The numbers of correct responses on the trials are added for a total immediate-memory recall score. After the third trial, the subject is read 24 words and asked to identify words contained in the original list. The number of incorrect responses is subtracted from the overall delayed recognition score.
DATA ANALYSIS
We calculated separate mixed-model (1 between, 1 within), repeated-measures analyses of variance (ANOVAs) for the SOT composite score, each of the 3 SOT ratio scores, the BESS score, and each of the neuropsychological test scores using SPSS 10.0 statistical software (SPSS, Inc, Chicago, IL). These analyses determined if significant differences existed between groups (injured and control) and across postinjury days for each of the dependent variables. An additional mixed-model repeated-measures ANOVA performed only on the injured group compared those subjects with LOC or amnesia or both against those subjects without LOC or amnesia across the same postinjury days. Tukey post hoc analyses were performed for all significant interactions. Level of significance (P < .05) was set a priori for all statistical analyses.
RESULTS
Seventy-two subjects participated in this study (50 males and 22 females). The injured group consisted of 36 Division I collegiate athletes (age = 19.5 ± 1.34 years, height = 180.34 ± 11.81 cm, weight = 83.43 ± 19.80 kg) who had sustained a concussion during either practice or competition. The control group consisted of 36 recreational and collegiate athletes (age = 20 ± 2.36 years, height = 179.07 ± 10.47 cm, weight = 81.50 ± 20.45 kg) who were matched with injured subjects for sex, age, height, weight, and activity level. The number of subjects experiencing signs and symptoms associated with concussion is presented in Table 2, and the sample is further described by sport in Table 3. All of the injured subjects were symptomatic at the time of injury, and all but 2 subjects were symptomatic on day 1 postinjury. Twenty-two subjects reported symptoms on day 3 postinjury, and only 12 subjects remained symptomatic beyond day 3 postinjury.
Table 2.
Frequency of Subjects Experiencing Signs and Symptoms After Injury (n = 36)
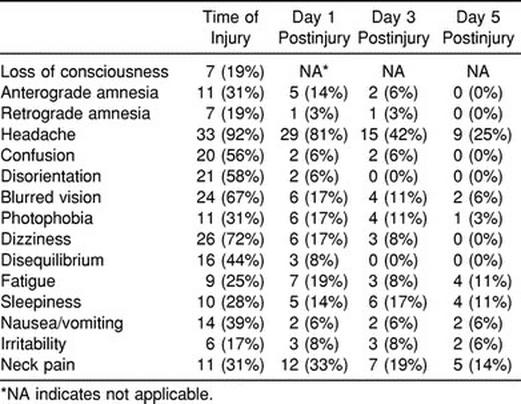
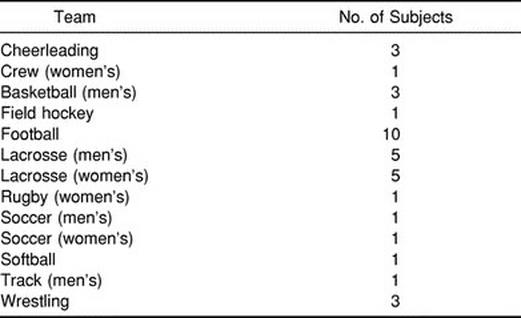
Table 3.
Frequency Distribution of Injured Subjects by Sport (n= 36)
Postural Stability Recovery
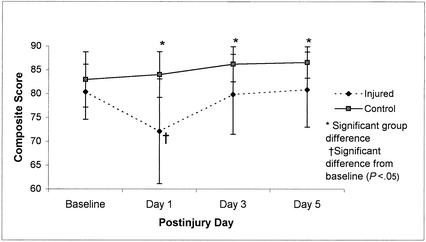
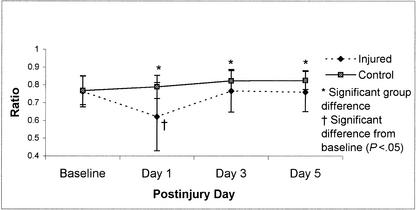
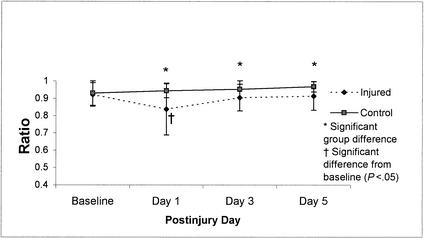
Repeated-measures ANOVA for SOT composite scores on the Smart Balance Master System revealed a significant group-by-day interaction (F3,210 = 10.17, P < .01). Tukey post hoc analysis revealed that injured subjects demonstrated decreased postural stability on day 1 postinjury in comparison with their baseline as well as in relation to the control subjects' postinjury day 1, 3, and 5 scores, respectively (Figure 4). While differences between control subjects and injured subjects were statistically significant on postinjury days 3 and 5, recovery back to baseline occurred between postinjury days 1 and 3 for the injured subjects. Additional analyses of the ratio scores (visual, vestibular, and somatosensory) revealed significant group-by-day interactions for the visual ratio (F3,210 = 13.57, P < .01) and vestibular ratio (F3,210 = 6.48, P < .01), suggesting that postural stability deficits observed in athletes with concussions could be linked to a sensory interaction problem. Mean scores for these ratios across the baseline and 3 postinjury test sessions are presented in Figures 5 and 6. Post hoc analyses again revealed low vestibular and visual ratios on day 1 postinjury in injured subjects compared with their baseline and postinjury day 3 as well as the control subjects' postinjury day 1, 3, and 5 ratio scores.
Figure 4.
Composite Score means (±SD) on the NeuroCom Smart Balance Master for 36 injured and 36 control subjects across test sessions (preseason through day 5 postinjury). Higher scores represent better performance.
Figure 5.
Vestibular ratio means (±SD) on the NeuroCom Smart Balance Master System for 36 injured and 36 control subjects across test sessions (preseason through day 5 postinjury). Higher scores represent better performance.
Figure 6.
Visual ratio means (±SD) on the NeuroCom Smart Balance Master for 36 injured and 36 control subjects across test sessions (preseason through day 5 postinjury). Higher scores represent better performance.
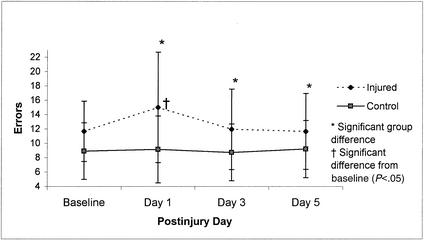
The BESS results revealed a similar trend to those of the SOT, as the repeated-measures ANOVA showed a significant group-by-day interaction (F3,210 = 2.68, P < .05). Tukey post hoc analysis again demonstrated that injured subjects had decreased postural stability on day 1 postinjury in comparison with baseline and day 3 postinjury scores as well as the control subjects' postinjury day 1, 3, and 5 scores (Figure 7).
Figure 7.
Balance Error Scoring System means (±SD) (combined errors on all 6 trials) for 36 injured and 36 control subjects across test sessions (preseason through day 5 postinjury). Lower scores represent better performance.
Neurocognitive Recovery
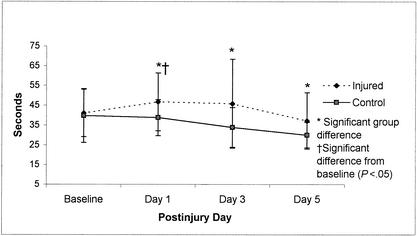
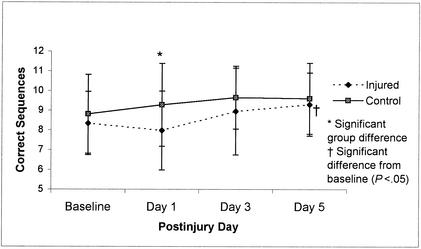
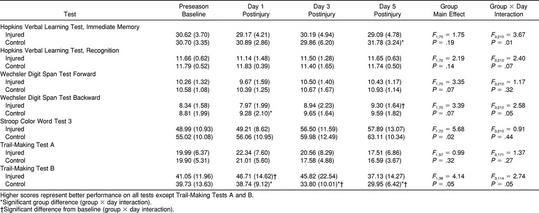
Repeated-measures ANOVA revealed significant group-by-day interactions for 3 of the neuropsychological tests; however, only the Trail-Making Test B (Figure 8) and Digit Span Test Backward (Figure 9) scores resulted in a recovery curve that could be logically associated with concussion and subsequent lowered neuropsychological performance. Results from these 2 tests revealed significant differences between the control group and injured group at postinjury days 1 (Trail-Making Test B and WDST) as well as postinjury days 3 and 5 (Trail-Making Test B) but failed to show a statistically significant decline in performance between baseline and postinjury scores. Despite the absence of this significant difference across days for the injured group, there is still a trend in the opposite direction from control subjects' scores at postinjury day 1, suggesting that injured subjects may have mild cognitive deficits during the initial days after injury on these 2 tests. Group comparisons for all neuropsychological test scores are presented in Table 4.
Figure 8.
Trail-Making Test B means (±SD) for 18 injured and 23 control subjects across test sessions (preseason through day 5 postinjury). Lower scores represent better performance.
Figure 9.
Wechsler Digit Span Test Backward means (±SD) (combined errors on all 6 trials) for 36 injured and 36 control subjects across test sessions (preseason through day 5 postinjury). Higher scores represent better performance.
Table 4.
Neuropsychological Test Results in Injured and Control Subjects: Mean (Standard Deviation)
Loss of Consciousness and Amnesia
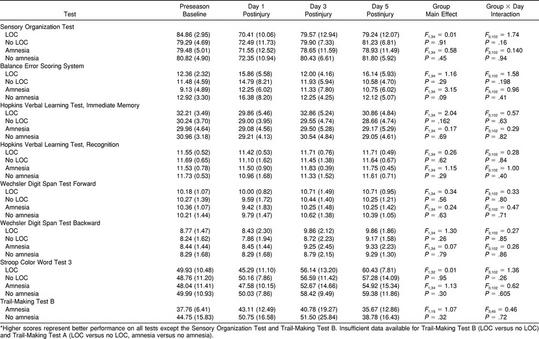
We compared injured subjects with (n = 12) and without (n = 24) amnesia and with (n = 7) and without (n = 29) LOC and found no significant group-by-day interactions or group main effects for either SOT or BESS scores (P > .05). The same analyses performed on neuropsychological test variables also revealed no significant interactions or main effects (P > .05) (Table 5). These findings indicate that injured athletes who experienced LOC or amnesia (or both) performed no worse than injured athletes who did not experience LOC or amnesia (or both) on any of the postural stability or neuropsychological tests.
Table 5.
Postural Stability and Neuropsychological Test Results in Athletes With and Without Loss of Consciousness (LOC) and Amnesia: Mean (Standard Deviation)*
DISCUSSION
The use of both neuropsychological screening and postural stability (balance) testing is gradually becoming standard practice in the management of sport-related concussion. However, much discussion continues among researchers and clinicians regarding the best test battery and test sequencing. Additionally, debate surrounds the emphasis placed on LOC and amnesia in the grading of concussion. Perhaps the most important finding in our investigation was that athletes recovering from cerebral concussion demonstrated postural stability deficits most likely linked to a sensory interaction problem during the immediate postinjury period. The initial 2 days after concussion appeared to be problematic for most concussed athletes, after which time they began to recover and eventually returned to their preseason postural stability baseline score around day 3 postinjury. The group differences found on postinjury days 3 and 5 are less important because baseline had been reached; however, an explanation might be that attention and concentration deficits prevented the subtle improvements that were observed in the control group with repeated testing. Future researchers will need to investigate days 2 and 4 postinjury to complete the recovery curve.
The overall postural stability deficit can best be explained by a sensory interaction problem that prevents concussed athletes from accurately using and exchanging sensory information from the visual, vestibular, and somatosensory systems. The integration of vestibular and visual information is essential for the maintenance of equilibrium under certain altered conditions similar to those performed during the SOT.43–47 If a subject has difficulty balancing under conditions in which environmental or sensory conditions (or both) have been altered, we can hypothesize that he or she is unable to appropriately integrate sensory input and select the most reliable cues for precise postural control. Examples might include the athlete's needing to make rapid postural adjustments in response to impact with the ground or other players as well as changes in reaction time associated with becoming fatigued. Occasionally, vision becomes obscured and range of motion becomes limited by protective headgear or other protective padding, requiring alternative sensory modalities to take control.
Recovery curves for the visual and vestibular ratios in Figures 5 and 6 suggest that the postinjury stability problems occurred primarily under the altered sensory conditions involving unstable (sway-referenced) surface conditions and either normal or absent vision (conditions 4 and 5). These results affirm our earlier findings of significant differences between concussed athletes and control subjects on day 1 postinjury compared with preseason or subsequent tests, or both, using the Chattecx Balance System1 (Chattanooga Group, Hixson, TN) and the NeuroCom Equitest.2 The current findings also concur with other recent investigations of patients with mild head or neck injuries. Such studies have revealed significantly higher magnitudes of postural instability during inaccurate visual conditions or altered surface conditions.40,41 Our findings on the BESS are also promising, given that they are consistent with an earlier finding of a significant relationship between SOT and BESS scores in a group of 16 concussed athletes.3 Based on our findings, the BESS is a practical, valid, and cost-effective method of objectively assessing postural stability in athletes suffering from concussion.
Much speculation exists about the underlying neural deficits causing postural instability after cerebral concussion. Some authors have suggested that the observed balance problems are diffuse and rarely related to pure vestibular and cerebellar dysfunction,37,38 while others claim that concussed individuals may need longer processing time to readjust balance because of slow subcortical activity and spatiotemporal disruption of postural responses.41 Minor axonal dysfunction at the level of the brainstem or cerebellum has also been proposed as the potential cause of postural instability after concussive injury.48 Although this may appear unlikely in sport-related concussion, the sudden deceleration of the brain causes shearing forces, which in turn cause transport failures in the axon.49 In other words, disruption of any axons responsible for transmitting information to centers responsible for maintenance of balance can lead to postural instability. Another possible explanation for postural stability or neurocognitive deficits after concussion could center on the biochemical cascade models that have been developed in animals. Hovda et al50 have described a neurochemical and metabolic cascade that occurs after cerebral concussion. Within the first hour of the concussion and up to several days postinjury, the brain is thought to be in a vulnerable state due to increased glucose metabolism and diminished cerebral blood flow. Although only speculative at this time, the imbalance between glucose needs and available supply during the first few days after injury could partially explain the deficits observed in our study.
Because the SOT cannot identify a specific lesion or pathologic condition, the source of concussion-related balance deficits is unknown. We know it will involve either peripheral sensory receptors responsible for detecting motion or central structures, primarily the cerebellum, cerebral cortex, and brain stem, which are involved in the perception and integration of sensory information. Some authors have reported that concussive trauma to the head or neck can result in changes to the normal weighing of sensory cues and create a reorganization pattern of the remaining sensory cues underlying postural control.51,52 The depressed visual and vestibular ratios found in our study may best be explained by this phenomenon.
The primary purposes of the human vestibular system are to (1) maintain the eyes fixed on a stationary target in the presence of head and body movement and (2) maintain balance in conjunction with additional information from visual and somatosensory inputs. To accomplish the first, the semicircular canals of the vestibular labyrinth sense angular acceleration of the head, converting it to velocity information and sending it through the vestibulo-ocular reflex pathways to the ocular muscles. Second, balance is maintained by central integration of vestibular, visual, and somatosensory orientation information. The vestibular system provides angular information from the semicircular canals and linear acceleration information (including gravity) from the utricles and saccules of the inner ear and transmits it via the vestibulospinal spinal tract to the spinal and lower extremity muscles. Under normal conditions, visual and somatosensory information is adequate for maintenance of balance; however, in populations with known vestibular deficits, the inner ear's sense of balance is essential when visual and somatosensory inputs are disrupted or provide conflicting information.
Two mechanisms are possible for vestibular involvement after cerebral concussion: (1) the peripheral receptors themselves may be damaged and provide inaccurate senses of motion or (2) the brain centers responsible for central integration of vestibular, visual, and somatosensory information may be impaired. Mallinson and Longridge53 found evidence of central integration balance deficits and subtle peripheral vestibular deficits when comparing SOT and electronystagmography results in patients with mild head injury from an associated whiplash injury. These findings suggest that various combinations of peripheral and central deficits may be the cause of balance deficits in athletes with concussion. An additional factor that surfaced from our research is the possibility that concentration and attention impairments identified on day 1 postinjury could be contributing factors to decreased postural stability. Future researchers should focus on this potential relationship.
Our findings did not substantiate the use of a long neuropsychological test battery for identifying underlying pathology in concussed athletes during the acute stage of injury. The injured athletes in this study demonstrated significantly poorer performance on selected tests of concentration and immediate memory recall (WDST Backward) and rapid visual processing and working memory (Trail-Making Test B). These 2 neuropsychological tests were the only ones to reveal significant differences relative to the control group at day 1 postinjury. Scores on the Trail-Making Test B also revealed group differences at postinjury days 3 and 5: the injured subjects did not appear to be learning the task as efficiently as the control subjects over the repeated tests. Therefore, understanding the learning curve may be as important as having a baseline measurement in cases in which improvement should be expected with repeated testing. Our research findings leave us with further questions surrounding the efficacy of certain neuropsychological testing during the acute stage of injury in athletes with relatively mild injuries. Is there really a cognitive deficit, or are these tests not sensitive enough to detect cognitive decline after this type of injury? While selected tests may be sensitive to the injury, others (such as the Trail-Making Test A, HVLT, Stroop Color Word Test 3, and WDST Forward) may not be as sensitive. In our study, the efficacy of the HVLT may be questioned in an athletic population given the sudden spreading of scores (between injured and control subjects) at day 5 postinjury. We propose that the motivation levels of many injured players by day 5 postinjury may explain the dropoff in HVLT scores. Several researchers have reported significant neuropsychological deficits during the acute stage of concussion,21–27 but the literature has failed to establish a consensus on which tests are most sensitive or which areas of cognition are most affected. Based on our findings, working memory, immediate memory recall, concentration, and rapid visual processing abilities appear to be affected during the initial stage of injury recovery. Interestingly, a previous study by our group found that the WDST and Trail-Making Test B were the best predictors of symptom severity at day 3 postinjury in collegiate football players.54
Some researchers suggest that neuropsychological testing may play a more important role in identifying undetected pathologic conditions once the athlete appears asymptomatic or in cases of postconcussion syndrome, when the athlete experiences lingering signs and symptoms.25,55,56 In analyzing our control subjects more closely, we found a very subtle learning curve between baseline and day 1 postinjury on the Stroop Color Word Test 3, the Trail Making Test B, and the WDST Backward, similar to that reported by Oliaro et al.57 As noted previously, the injured subjects in our study demonstrated a deviation from this learning curve, as they did not learn at the same rate as control subjects on these 2 tests. Injured subjects also had heightened variability among subjects (larger standard deviations) on several of the neuropsychological tests on day 1 postinjury, further supporting our claim that they probably had mild cognitive deficits. Echemendia et al28 reported similar learning trends and suggested that a return to preseason baseline score may not be a sufficient indicator of “normal” functioning. Our findings support their claim that injured players should probably be expected to exceed baseline scores on measures with known practice effects before they are rendered “normal.”
The recovery for signs and symptoms (Table 2) appears to follow the recovery for postural stability and selected tests of neurocognitive function. With the exception of headache, most signs and symptoms had resolved by day 3 postinjury. However, 42% of the athletes still reported a headache on day 3 postinjury, and 25% reported a headache on day 5 postinjury. While our purpose was not to study RTP decision making, most players had returned to restricted participation by day 5 postinjury. Only those with lingering symptomatology were withheld beyond day 10 postinjury. When the injured subjects were stratified by LOC and amnesia, no significant interactions or group main effects (Table 5) were seen for any of the postural stability or neuropsychological measures. This finding suggests that neither LOC nor amnesia was associated with course of recovery or was a predictor of postural stability or neuropsychological test performance during the acute stage of injury. Our findings are consistent with those of Lovell et al,58 who reported no neuropsychological deficits in patients with traumatic LOC compared with those without LOC or those who were uncertain about LOC. Clinicians should, therefore, be cautious about overemphasizing LOC and amnesia while potentially ignoring other important signs and symptoms.
CONCLUSIONS
No two concussions are created equal. Some injuries may involve the cognitive centers of the brain, while others involve the balance centers. The motor domain of neurologic functioning should, therefore, be assessed along with the cognitive domain after all concussions. Our findings reveal that postural stability deficits were present in most athletes sustaining cerebral concussion, and often they do not resolve until 3 days postinjury. It appears that sensory feedback from the visual, vestibular, and somatosensory systems in athletes with concussion is not properly processed during the first few days after injury. Although neuropsychological testing has become popular in recent years for assessing the cognitive domain of neurologic functioning, more research is necessary to establish the most sensitive, practical, and useful battery of tests. Of the tests we used in this study, the WDST Backward and the Trail-Making Test B were most sensitive for tracking recovery after cerebral concussion in athletes. Memory and concentration deficits during the immediate recovery period can be detected using these tests. One of the limitations of our study is that it involved subjects with relatively mild concussions (grades 1 and 2), most of whom had not experienced previous concussions. Future researchers should attempt to compare the neuropsychological and postural stability recovery curves in athletes with more serious and recurrent episodes of concussion.
In the absence of sophisticated forceplate systems, the BESS is an efficient and cost-effective alternative for objectively assessing postural stability. Similarly, the clinician who does not have access to traditional neuropsychological assessment tools should at the very least find simple and easy-to-use alternatives, such as the Standardized Assessment of Concussion.30,31 Once validated in the athletic setting, computerized neuropsychological assessment tools may become a more useful option for the sports medicine clinician. Finally, we recommend that athletes sustaining a concussion never be permitted to return to activity until all postconcussive symptoms have resolved. Based on our findings, athletes whose symptoms resolve quickly after injury should, at the very least, be held from competition for 3 days after any episode in which they might have sustained a concussion. Clinicians should seriously consider whether or not they might be placing athletes at risk by returning them earlier than 3 days postinjury. Clinicians should also realize that postural stability and neuropsychological testing are only 2 pieces of a very large puzzle in the assessment of concussion. Concussive injury may not necessarily affect the postural control system or neurocognitive areas of the brain in every patient. Furthermore, the presence of LOC or amnesia or both may have little to do with the recovery rate, and therefore, these conditions should not be overemphasized in the management of concussion. The most comprehensive concussion assessment will involve a sound clinical examination with close monitoring of all symptoms while including objective measurements such as postural stability testing and neuropsychological testing.
ACKNOWLEDGMENTS
We thank James Onate, David Brunken, Jason Scibek, Thomas Michell, Darin Padua, and Bryan Riemann for assisting in data collection. This research was funded in part by grants from the National Athletic Trainers' Association Research and Education Foundation, the National Operating Committee for Standards in Athletic Equipment, and the Injury Prevention Research Center, University of North Carolina. We thank the agencies for their support of these projects.
REFERENCES
- 1.Guskiewicz KM, Riemann BL, Perrin DH, Nashner LM. Alternative approaches to the assessment of mild head injury in athletes. Med Sci Sports Exerc. 1997;29:S213–S221. doi: 10.1097/00005768-199707001-00003. [DOI] [PubMed] [Google Scholar]
- 2.Guskiewicz KM, Perrin DH, Gansneder BM. Effect of mild head injury on postural stability in athletes. J Athl Train. 1996;31:300–306. [PMC free article] [PubMed] [Google Scholar]
- 3.Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35:19–25. [PMC free article] [PubMed] [Google Scholar]
- 4.Cantu RC. Second-impact syndrome. Clin Sports Med. 1998;17:37–44. doi: 10.1016/s0278-5919(05)70059-4. [DOI] [PubMed] [Google Scholar]
- 5.Fekete JF. Severe brain injury and death following minor hockey accidents: the effectiveness of the “safety helmets” of amateur hockey players. Can Med Assoc J. 1968;99:1234–1239. [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly JP, Nichols JS, Filley CM, Lillehi KO, Rubinstein OD, Kleinschmidt-DeMasters BK. Concussion in sports: guidelines for the prevention of catastrophic outcome. JAMA. 1991;266:2867–2869. doi: 10.1001/jama.266.20.2867. [DOI] [PubMed] [Google Scholar]
- 7.McCrory PR, Berkovic SF. Second impact syndrome. Neurology. 1998;50:677–683. doi: 10.1212/wnl.50.3.677. [DOI] [PubMed] [Google Scholar]
- 8.Saunders RL, Harbaugh RE. The second impact in catastrophic contact-sports head trauma. JAMA. 1984;252:538–539. [PubMed] [Google Scholar]
- 9.Jordan BD. Proceedings of the Mild Brain Injury in Sports Summit. Dallas, TX: National Athletic Trainers' Association, Inc; 1994. Sports injuries; pp. 43–46. [Google Scholar]
- 10.Ommaya AK, Ommaya AK, Salazar AM. Proceedings of the Mild Brain Injury in Sports Summit. Dallas, TX: National Athletic Trainers' Association Inc; 1994. A spectrum of mild brain injuries in sports; pp. 72–80. [Google Scholar]
- 11.Powell JW, Barber-Foss KD. Traumatic brain injury in high school athletes. JAMA. 1999;282:958–963. doi: 10.1001/jama.282.10.958. [DOI] [PubMed] [Google Scholar]
- 12.Albright JP, McAuley E, Martin RK, Crowley ET, Foster DT. Head and neck injuries in college football: an eight-year analysis. Am J Sports Med. 1985;13:147–152. doi: 10.1177/036354658501300301. [DOI] [PubMed] [Google Scholar]
- 13.Buckley WE. Concussions in college football: a multivariate analysis. Am J Sports Med. 1988;16:51–56. doi: 10.1177/036354658801600109. [DOI] [PubMed] [Google Scholar]
- 14.Dick RW. 1997–98 NCAA Injury Surveillance System. Mission, KS: National Collegiate Athletic Association; 1998. Football injury report. [Google Scholar]
- 15.Guskiewicz KM, Weaver NL, Padua DA, Garrett WE., Jr Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28:643–650. doi: 10.1177/03635465000280050401. [DOI] [PubMed] [Google Scholar]
- 16.Collins MW, Grindel SH, Lovell MR, et al. Relationship between concussion and neuropsychological performance in college football players. JAMA. 1999;282:964–970. doi: 10.1001/jama.282.10.964. [DOI] [PubMed] [Google Scholar]
- 17.Matser EJT, Kessels AG, Jordan BD, Lezak MD, Troost J. Chronic traumatic brain injury in professional soccer players. Neurology. 1998;51:791–796. doi: 10.1212/wnl.51.3.791. [DOI] [PubMed] [Google Scholar]
- 18.Matser EJT, Kessels AG, Lezak MD, Jordan BD, Troost J. Neuropsychological impairment in amateur soccer players. JAMA. 1999;282:971–973. doi: 10.1001/jama.282.10.971. [DOI] [PubMed] [Google Scholar]
- 19.Tysvaer AT, Storli OV, Bachen NI. Soccer injuries to the brain: a neurologic and electroencephalographic study of former players. Acta Neurol Scand. 1989;80:151–156. doi: 10.1111/j.1600-0404.1989.tb03858.x. [DOI] [PubMed] [Google Scholar]
- 20.Tysvaer AT, Løchen EA. Soccer injuries to the brain: a neuropsychologic study of former players. Am J Sports Medicine. 1991;19:56–60. doi: 10.1177/036354659101900109. [DOI] [PubMed] [Google Scholar]
- 21.Barth J, Alves W, Ryan T, et al. Mild head injury in sports: neuropsychological sequela and recovery of function. In: Levin H, Eisenberg H, Benton A, editors. Mild Head Injury. New York: Oxford University Press; 1989. pp. 257–275. [Google Scholar]
- 22.Gentilini M, Nichelli P, Schoenhuber R, et al. Neuropsychological evaluation of mild head injury. J Neurol Neurosurg Psychiatry. 1985;48:137–140. doi: 10.1136/jnnp.48.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gronwall D, Wrightson P. Cumulative effect of concussion. Lancet. 1975;2:995–997. doi: 10.1016/s0140-6736(75)90288-3. [DOI] [PubMed] [Google Scholar]
- 24.Gronwall D, Wrightson P. Delayed recovery of intellectual function after minor head injury. Lancet. 1974;14:605–609. doi: 10.1016/s0140-6736(74)91939-4. [DOI] [PubMed] [Google Scholar]
- 25.Leininger BE, Gramling SE, Farrell AD, Kreutzer JS, Peck EA., 3d Neuropsychological deficits in symptomatic minor head injury patients after concussion and mild concussion. J Neurol Neurosurg Psychiatry. 1990;53:293–296. doi: 10.1136/jnnp.53.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin HS, Williams DH, Eisenberg HM, High WM, Jr, Guinto FC., Jr Serial MRI and neurobehavioral findings after mild to moderate closed head injury. J Neurol Neurosurg Psychiatry. 1992;55:255–262. doi: 10.1136/jnnp.55.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macciocchi SN, Barth JT, Alves W, Rimel RW, Jane JA. Neuropsychological functioning and recovery after mild head injury in collegiate athletes. Neurosurgery. 1996;39:510–514. [PubMed] [Google Scholar]
- 28.Echemendia RJ, Putukian M, Mackin RS, Julian L, Shoss N. Neuropsychological test performance prior to and following sports-related mild traumatic brain injury. Clin J Sport Med. 2001;11:23–31. doi: 10.1097/00042752-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Rimel RW, Giordani B, Barth JT, Boll TJ, Jane JA. Disability caused by minor head injury. Neurosurgery. 1981;9:221–228. [PubMed] [Google Scholar]
- 30.McCrea M, Kelly JP, Kluge J, Ackley B, Randolph C. Standardized assessment of concussion in football players. Neurology. 1997;48:586–588. doi: 10.1212/wnl.48.3.586. [DOI] [PubMed] [Google Scholar]
- 31.McCrea M, Kelly JP, Randolph C, et al. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J Head Trauma Rehabil. 1998;13:27–35. doi: 10.1097/00001199-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Adams J. The neuropathology of head injuries. In: Vinken P, Bruyn G, editors. Handbook of Clinical Neurology: Injuries of the Brain and Skull. Amsterdam, Netherlands: North-Holland Publishing Co; 1975. pp. 35–65. [Google Scholar]
- 33.Arcan M, Brull MA, Najenson T, Solzi P. FGP assessment of postural disorders during the process of rehabilitation. Scand J Rehabil Med. 1977;9:165–168. [PubMed] [Google Scholar]
- 34.Mauritz KH, Dichgans J, Hufschmidt A. Quantitative analysis of stance in late cortical cerebellar atrophy of the anterior lobe and other forms of cerebellar ataxia. Brain. 1979;102:461–482. doi: 10.1093/brain/102.3.461. [DOI] [PubMed] [Google Scholar]
- 35.McIntosh T, Vink R. Biomechanical and pathophysiologic mechanisms in experimental mild to moderate traumatic brain injury. In: Hoff J, Anderson T, Cole T, editors. Mild to Moderate Head Injury. Boston, MA: Blackwell Scientific Publications; 1989. pp. 135–145. [Google Scholar]
- 36.Mitchell DE, Adams JH. Primary focal impact damage to the brainstem in blunt head injuries: does it exist? Lancet. 1973;2:215–218. doi: 10.1016/s0140-6736(73)93128-0. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann JF, Boswell S, Price R, et al. Quantitative evaluation of sway as an indicator of functional balance in post-traumatic brain injury. Arch Phys Med Rehabil. 1990;71:955–962. [PubMed] [Google Scholar]
- 38.Shumway-Cook A, Olmscheid R. A systems analysis of postural dyscontrol in traumatically brain-injured patients. J Head Trauma Rehabil. 1990;5:51–62. [Google Scholar]
- 39.Wober C, Oder W, Kollegger H, et al. Posturographic measurement of body sway in survivors of severe closed head injury. Arch Phys Med Rehabil. 1993;74:1151–1156. [PubMed] [Google Scholar]
- 40.Rubin AM, Woolley SM, Dailey VM, Goebel JA. Postural stability following mild head or whiplash injuries. Am J Otology. 1995;16:216–221. [PubMed] [Google Scholar]
- 41.Geurts ACH, Ribbers GM, Knoop JA, van Limbeek J. Identification of static and dynamic postural instability following traumatic brain injury. Arch Phys Med Rehabil. 1996;77:639–644. doi: 10.1016/s0003-9993(96)90001-5. [DOI] [PubMed] [Google Scholar]
- 42.Riemann BL, Guskiewicz KM, Shields E. Relationship between clinical and forceplate measures of postural stability. J Sport Rehabil. 1999;8:71–82. [Google Scholar]
- 43.Nashner L. Adaptation of human movement to altered environments. Trends Neurosci. 1982;5:358–361. [Google Scholar]
- 44.Nashner LM, Black FO, Wall C., 3d Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J Neurosci. 1982;2:536–544. doi: 10.1523/JNEUROSCI.02-05-00536.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction on balance: suggestion from the field. Phys Ther. 1986;66:1548–1550. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 46.Horstmann GA, Dietz V. The contribution of vestibular input to the stabilization of human posture: a new experimental approach. Neurosci Lett. 1988;95:179–184. doi: 10.1016/0304-3940(88)90653-2. [DOI] [PubMed] [Google Scholar]
- 47.Norre M. Sensory interaction testing in platform posturography. J Laryngol Otol. 1993;107:496–501. doi: 10.1017/s0022215100123564. [DOI] [PubMed] [Google Scholar]
- 48.Blumbergs PC, Jones NR, North JB. Diffuse axonal injury in head trauma. J Neurol Neurosurg Psychiatry. 1989;52:838–841. doi: 10.1136/jnnp.52.7.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexander MP. Mild traumatic brain injury pathophysiology, natural history, and clinical management. Neurology. 1995;45:1253–1260. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- 50.Hovda DA, Prins M, Becker DP, et al. Neurobiology of concussion. In: Bailes JE, Lovell MR, Maroon JC, editors. Sports-Related Concussion. St Louis, MO: Quality Medical Publishing, Inc; 1999. pp. 12–51. [Google Scholar]
- 51.Brown JJ. A systematic approach to the dizzy patient. Neurol Clin. 1990;8:209–224. [PubMed] [Google Scholar]
- 52.Droulez J, Berthoz A, Vidal PP. Use and limits of visual vestibular interaction in the control of posture. In: Igarashi M, Block FO, editors. Vestibular and Visual Control of Posture and Locomotor Equilibrium. Basel, Switzerland: Karger; 1985. pp. 14–21. [Google Scholar]
- 53.Mallinson AI, Longridge NS. Dizziness from whiplash and head injury: differences between whiplash and head injury. Am J Otol. 1998;19:814–818. [PubMed] [Google Scholar]
- 54.Ross SE, Guskiewicz KM, Onate JA. Symptomatology following cerebral concussion and its relationship with neuropsychological and postural stability tests [abstract] J Athl Train. 2000;35:S53. [Google Scholar]
- 55.Bohnen N, Jolles J. Neurobehavioral aspects of postconcussive symptoms after mild head injury. J Nerv Ment Dis. 1992;180:683–692. doi: 10.1097/00005053-199211000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Bohnen N, Twijnstra A, Jolles J. Performance in the Stroop Color Word Test in relationship to the persistence of symptoms following mild head injury. Acta Neurol Scand. 1992;85:116–121. doi: 10.1111/j.1600-0404.1992.tb04009.x. [DOI] [PubMed] [Google Scholar]
- 57.Oliaro S, Guskiewicz KM, Prentice WE. Establishment of normative data on cognitive tests for comparison with athletes sustaining mild head injury. J Athl Train. 1998;33:36–40. [PMC free article] [PubMed] [Google Scholar]
- 58.Lovell MR, Iverson GL, Collins MW, McKeag D, Maroon J. Does loss of consciousness predict neuropsychological decrements after concussion? Clin J Sport Med. 1999;9:193–198. doi: 10.1097/00042752-199910000-00002. [DOI] [PubMed] [Google Scholar]