Abstract
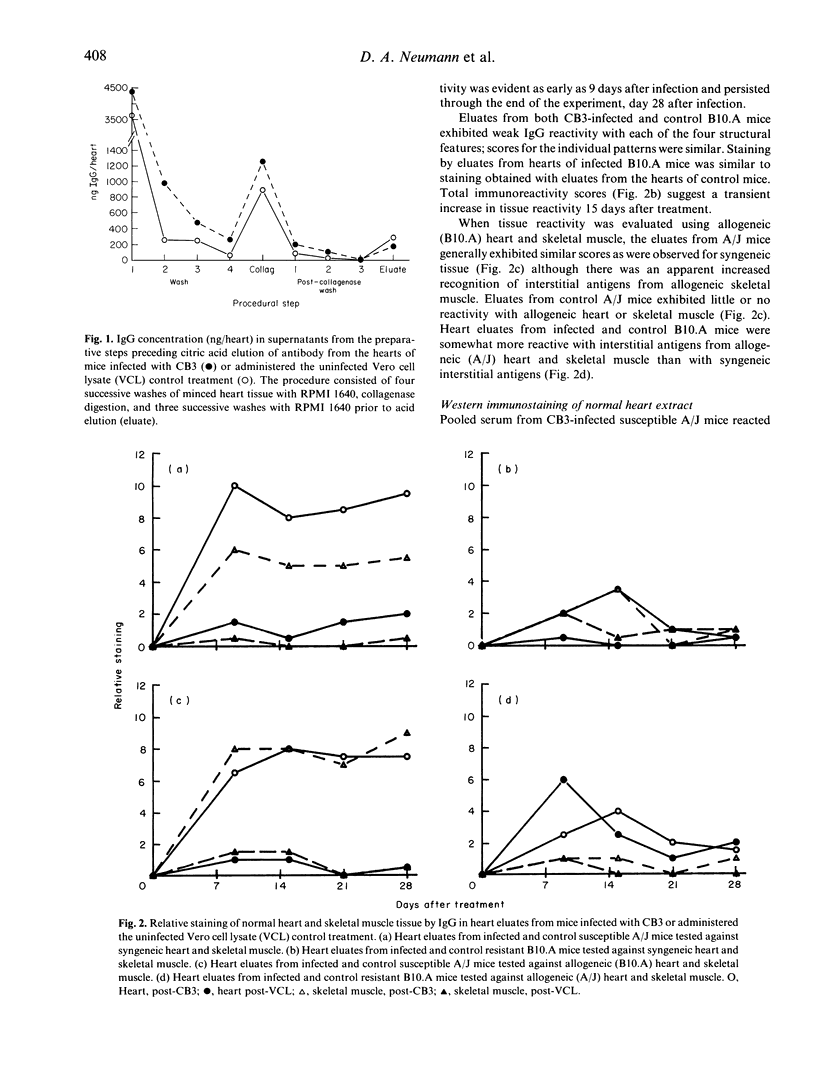
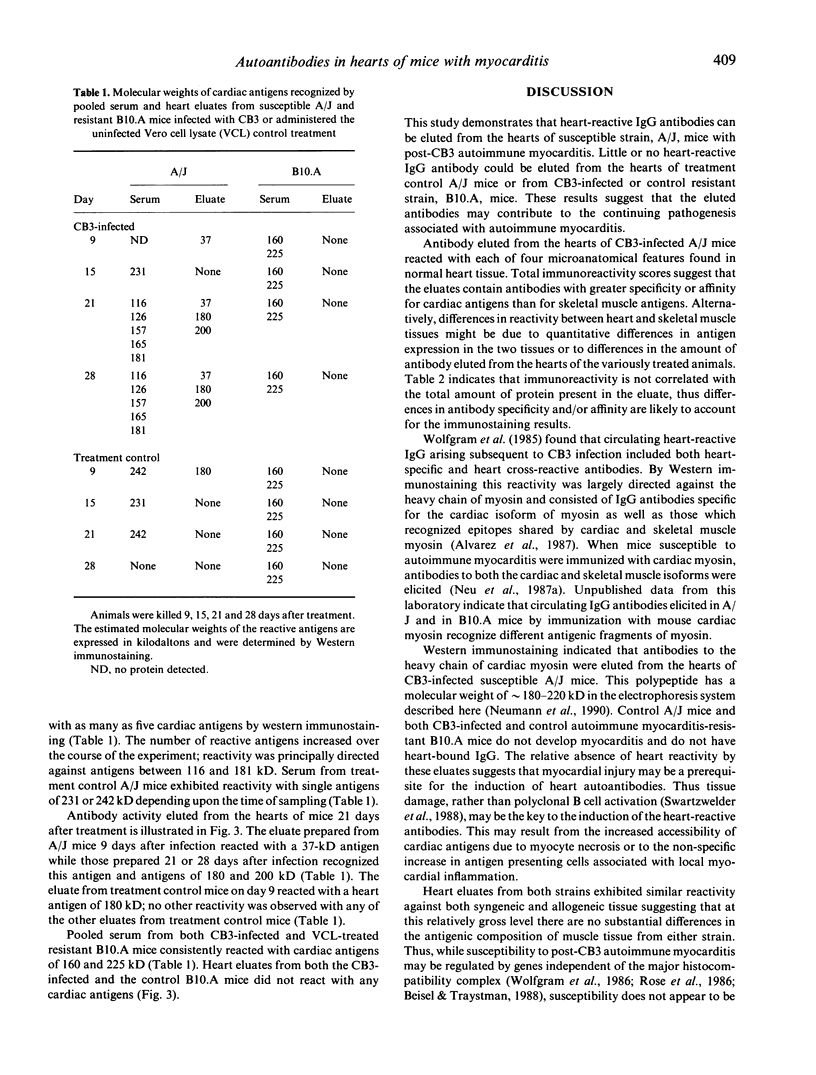
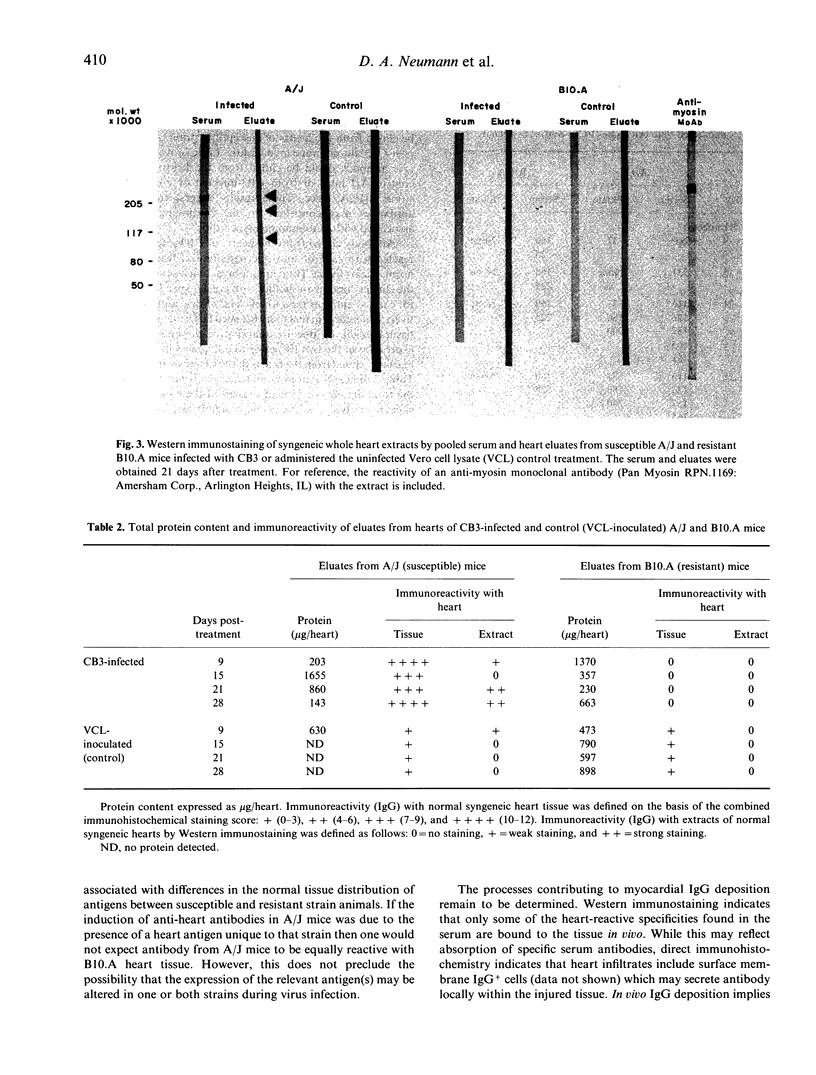
This study was undertaken to determine if immunoglobulin G (IgG) antibodies could be eluted from the hearts of mice with Coxsackievirus B3-induced autoimmune myocarditis and to characterize the immunoreactivity of any elutable autoantibodies. Susceptible (A/J) and resistant (B10.A) mice were administered the virus or the control treatment and killed at various times after treatment. Acid eluates from pooled heart tissue from each treatment group and each time were tested for IgG reactivity with normal heart tissue by immunohistochemistry and with normal heart extracts by Western immunostaining. Eluates from infected A/J mice reacted strongly with syngeneic heart and modestly with syngeneic skeletal muscle tissue. Eluates from infected B10.A or control mice of either strain exhibited little reactivity with either tissue. Tissue reactivity was similar when allogeneic tissue was used as the substrate. Eluates from infected A/J mice recognized the heavy chain of cardiac myosin and several other cardiac antigens by Western immunostaining while eluates from the other treatment groups exhibited little or no reactivity with any normal heart constituents. These results indicate that in vivo IgG deposition occurs in the hearts of mice with post-infectious autoimmune myocarditis and that the specificity of these antibodies is similar to that reported for serum from animals with this disease. The mechanism(s) leading to myocardial IgG deposition and its possible role in pathogenesis remain to be elucidated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez F. L., Neu N., Rose N. R., Craig S. W., Beisel K. W. Heart-specific autoantibodies induced by Coxsackievirus B3: identification of heart autoantigens. Clin Immunol Immunopathol. 1987 Apr;43(1):129–139. doi: 10.1016/0090-1229(87)90164-4. [DOI] [PubMed] [Google Scholar]
- Anhalt G. J., Labib R. S., Voorhees J. J., Beals T. F., Diaz L. A. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982 May 20;306(20):1189–1196. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- Calder E. A., McLeman D., Irvine W. J. Lymphocyte cytotoxicity induced by pre-incubation with serum from patients with Hashimoto thyroiditis. Clin Exp Immunol. 1973 Nov;15(3):467–470. [PMC free article] [PubMed] [Google Scholar]
- Daar A. S., Fabre J. W. Organ-specific IgM autoantibodies to liver, heart and brain in man: generalized occurrence and possible functional significance in normal individuals, and studies in patients with multiple sclerosis. Clin Exp Immunol. 1981 Jul;45(1):37–47. [PMC free article] [PubMed] [Google Scholar]
- Davis P., Percy J. S., Russell A. S. Correlation between levels of DNA antibodies and clinical disease activity in SLE. Ann Rheum Dis. 1977 Apr;36(2):157–159. doi: 10.1136/ard.36.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giometti C. S., Anderson N. G., Tollaksen S. L., Edwards J. J., Anderson N. L. Analytical techniques for cell fractions. XXVII. Use of heart proteins as reference standards in two-dimensional electrophoresis. Anal Biochem. 1980 Feb;102(1):47–58. doi: 10.1016/0003-2697(80)90315-2. [DOI] [PubMed] [Google Scholar]
- Granholm N. A., Graves K., Izui S., Cavallo T. Pathogenic role of anti-DNA antibodies in murine lupus nephritis. J Clin Lab Immunol. 1985 Nov;18(3):113–118. [PubMed] [Google Scholar]
- Guilbert B., Dighiero G., Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982 Jun;128(6):2779–2787. [PubMed] [Google Scholar]
- Hawkins B. R., Cheah P. S., Dawkins R. L., Whittingham S., Burger H. G., Patel Y., Mackay I. R., Welborn T. A. Diagnostic significance of thyroid microsomal antibodies in randomly selected population. Lancet. 1980 Nov 15;2(8203):1057–1059. doi: 10.1016/s0140-6736(80)92276-x. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Heintz N., Tracy R. Coxsackievirus B-3-induced myocarditis. Virus and actinomycin D treatment of myocytes induces novel antigens recognized by cytolytic T lymphocytes. J Immunol. 1988 Nov 1;141(9):3214–3219. [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis. Identification of different pathogenic mechanisms in DBA/2 and Balb/c mice. Am J Pathol. 1986 Feb;122(2):284–291. [PMC free article] [PubMed] [Google Scholar]
- Jaroszewski J., Sundick R. S., Rose N. R. Effects of antiserum containing thyroglobulin antibody on the chicken thyroid gland. Clin Immunol Immunopathol. 1978 May;10(1):95–103. doi: 10.1016/0090-1229(78)90013-2. [DOI] [PubMed] [Google Scholar]
- Jasin H. E. Autoantibody specificities of immune complexes sequestered in articular cartilage of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1985 Mar;28(3):241–248. doi: 10.1002/art.1780280302. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Sorensen K., Wong P., Bolton P. Antigenicity, storage, and aging: physiologic autoantibodies to cell membrane and serum proteins and the senescent cell antigen. Mol Cell Biochem. 1982 Nov 26;49(2):65–85. doi: 10.1007/BF00242486. [DOI] [PubMed] [Google Scholar]
- Mach P. S., Kharouby M., Lutcher F., Olivier J. L., Bazely N., Dougados M., Amor B. The in vitro production and regulation of anti-double stranded DNA antibodies by peripheral blood mononuclear cells from normals and patients with systemic lupus erythematosus. Clin Exp Immunol. 1984 Aug;57(2):338–344. [PMC free article] [PubMed] [Google Scholar]
- Mahi-Brown C. A., Yule T. D., Tung K. S. Adoptive transfer of murine autoimmune orchitis to naive recipients with immune lymphocytes. Cell Immunol. 1987 May;106(2):408–419. doi: 10.1016/0008-8749(87)90183-3. [DOI] [PubMed] [Google Scholar]
- Male D., Roitt I. M., Hay F. C. Analysis of immune complexes in synovial effusions of patients with rheumatoid arthritis. Clin Exp Immunol. 1980 Feb;39(2):297–306. [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Sano H., Abe T., Homma M., Steinberg A. D. Correlation between clinical activity of systemic lupus erythematosus and the amounts of DNA in DNA/anti-DNA antibody immune complexes. J Immunol. 1982 Nov;129(5):1960–1965. [PubMed] [Google Scholar]
- Neu N., Beisel K. W., Traystman M. D., Rose N. R., Craig S. W. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to Coxsackievirus B3-induced myocarditis. J Immunol. 1987 Apr 15;138(8):2488–2492. [PubMed] [Google Scholar]
- Neu N., Craig S. W., Rose N. R., Alvarez F., Beisel K. W. Coxsackievirus induced myocarditis in mice: cardiac myosin autoantibodies do not cross-react with the virus. Clin Exp Immunol. 1987 Sep;69(3):566–574. [PMC free article] [PubMed] [Google Scholar]
- Neumann D. A., Burek C. L., Baughman K. L., Rose N. R., Herskowitz A. Circulating heart-reactive antibodies in patients with myocarditis or cardiomyopathy. J Am Coll Cardiol. 1990 Nov;16(6):839–846. doi: 10.1016/s0735-1097(10)80331-6. [DOI] [PubMed] [Google Scholar]
- Pankewycz O. G., Migliorini P., Madaio M. P. Polyreactive autoantibodies are nephritogenic in murine lupus nephritis. J Immunol. 1987 Nov 15;139(10):3287–3294. [PubMed] [Google Scholar]
- Roscoe J. T., Diaz L., Sampaio S. A., Castro R. M., Labib R. S., Takahashi Y., Patel H., Anhalt G. J. Brazilian pemphigus foliaceus autoantibodies are pathogenic to BALB/c mice by passive transfer. J Invest Dermatol. 1985 Dec;85(6):538–541. doi: 10.1111/1523-1747.ep12277362. [DOI] [PubMed] [Google Scholar]
- Rose N. R., Wolfgram L. J., Herskowitz A., Beisel K. W. Postinfectious autoimmunity: two distinct phases of coxsackievirus B3-induced myocarditis. Ann N Y Acad Sci. 1986;475:146–156. doi: 10.1111/j.1749-6632.1986.tb20864.x. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stefansson K., Marton L. S., Dieperink M. E., Molnar G. K., Schlaepfer W. W., Helgason C. M. Circulating autoantibodies to the 200,000-dalton protein of neurofilaments in the serum of healthy individuals. Science. 1985 May 31;228(4703):1117–1119. doi: 10.1126/science.4039466. [DOI] [PubMed] [Google Scholar]
- Swartzwelder F. J., Barua P. K., Albini B., Stinson M. W. Heart-reactive antibodies in rabbit anti-Streptococcus mutans sera fail to cross-react with Streptococcus mutans. J Immunol. 1988 Feb 1;140(3):954–961. [PubMed] [Google Scholar]
- Taurog J. D., Sandberg G. P., Mahowald M. L. The cellular basis of adjuvant arthritis. II. Characterization of the cells mediating passive transfer. Cell Immunol. 1983 Aug;80(1):198–204. doi: 10.1016/0008-8749(83)90106-5. [DOI] [PubMed] [Google Scholar]
- Trentham D. E., Dynesius R. A., David J. R. Passive transfer by cells of type II collagen-induced arthritis in rats. J Clin Invest. 1978 Aug;62(2):359–366. doi: 10.1172/JCI109136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladutiu A. O., Rose N. R. Transfer of experimental autoimmune thyroiditis of the mouse by serum. J Immunol. 1971 Apr;106(4):1139–1142. [PubMed] [Google Scholar]
- Williams W. V., Kyriakos M., Sharp G. C., Braley-Mullen H. Augmentation of transfer of experimental autoimmune thyroiditis (EAT) in mice by irradiation of recipients. Cell Immunol. 1987 Oct 15;109(2):397–406. doi: 10.1016/0008-8749(87)90322-4. [DOI] [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Herskowitz A., Rose N. R. Variations in the susceptibility to Coxsackievirus B3-induced myocarditis among different strains of mice. J Immunol. 1986 Mar 1;136(5):1846–1852. [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Rose N. R. Heart-specific autoantibodies following murine coxsackievirus B3 myocarditis. J Exp Med. 1985 May 1;161(5):1112–1121. doi: 10.1084/jem.161.5.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]



