Abstract
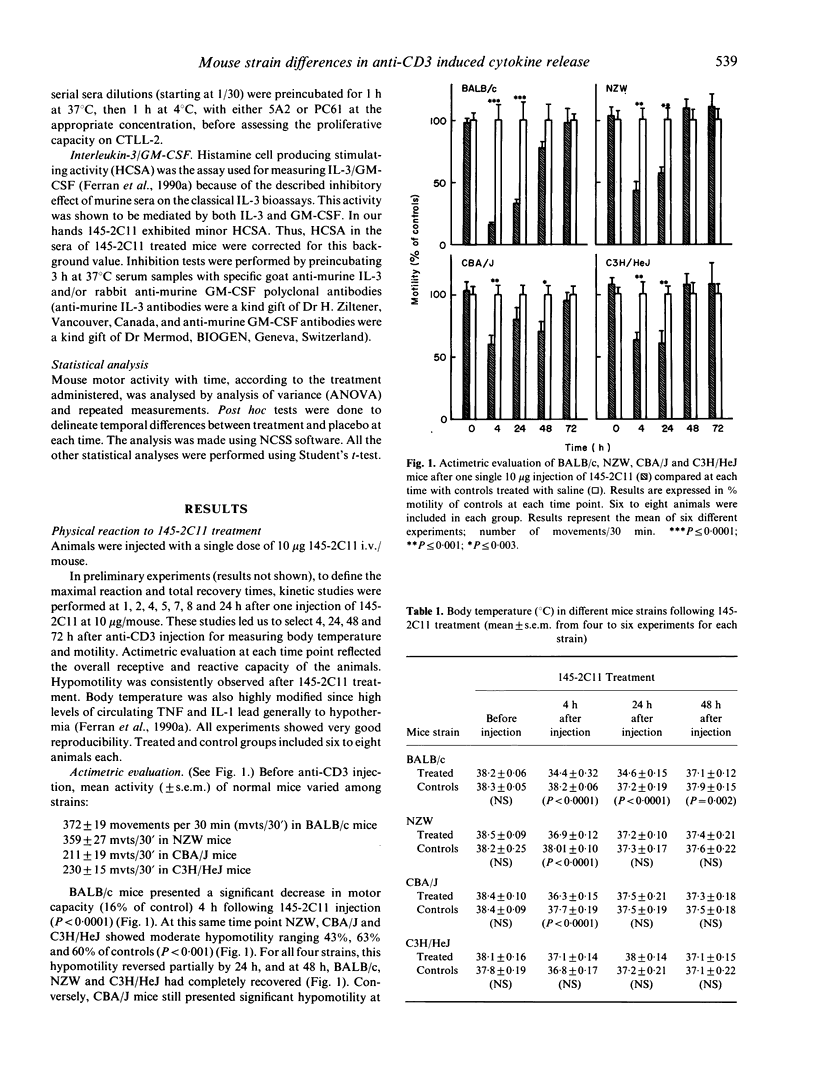
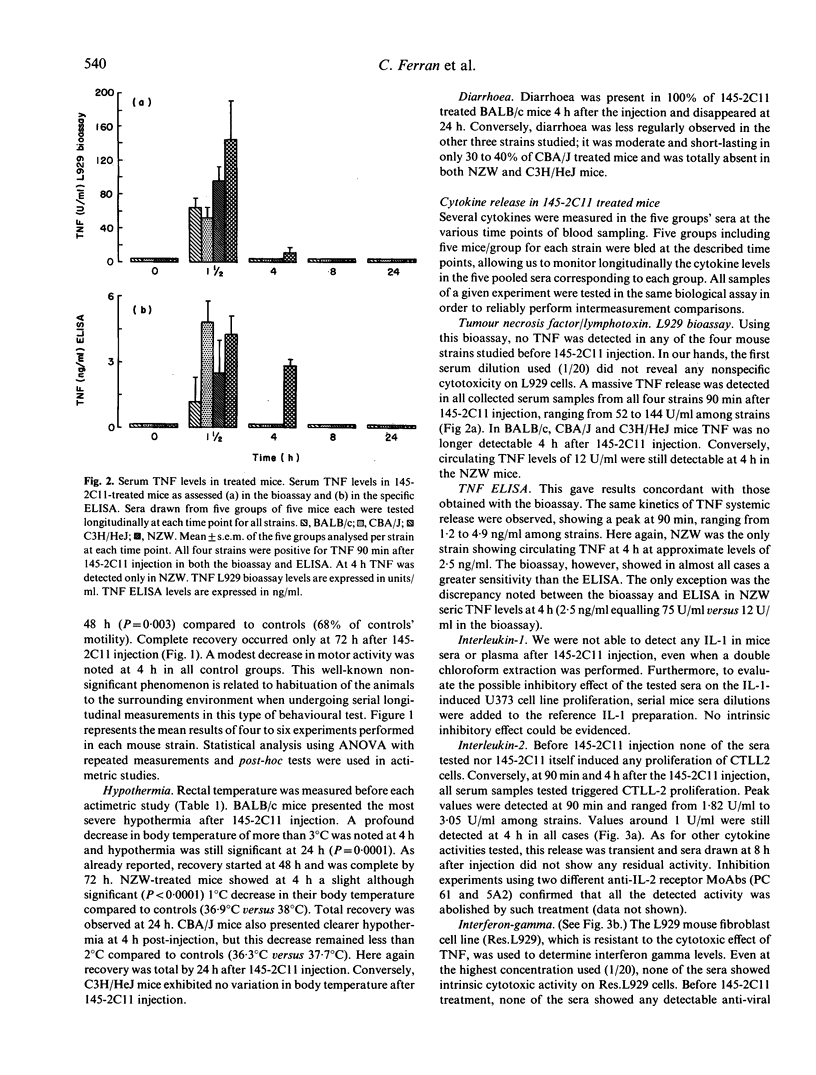
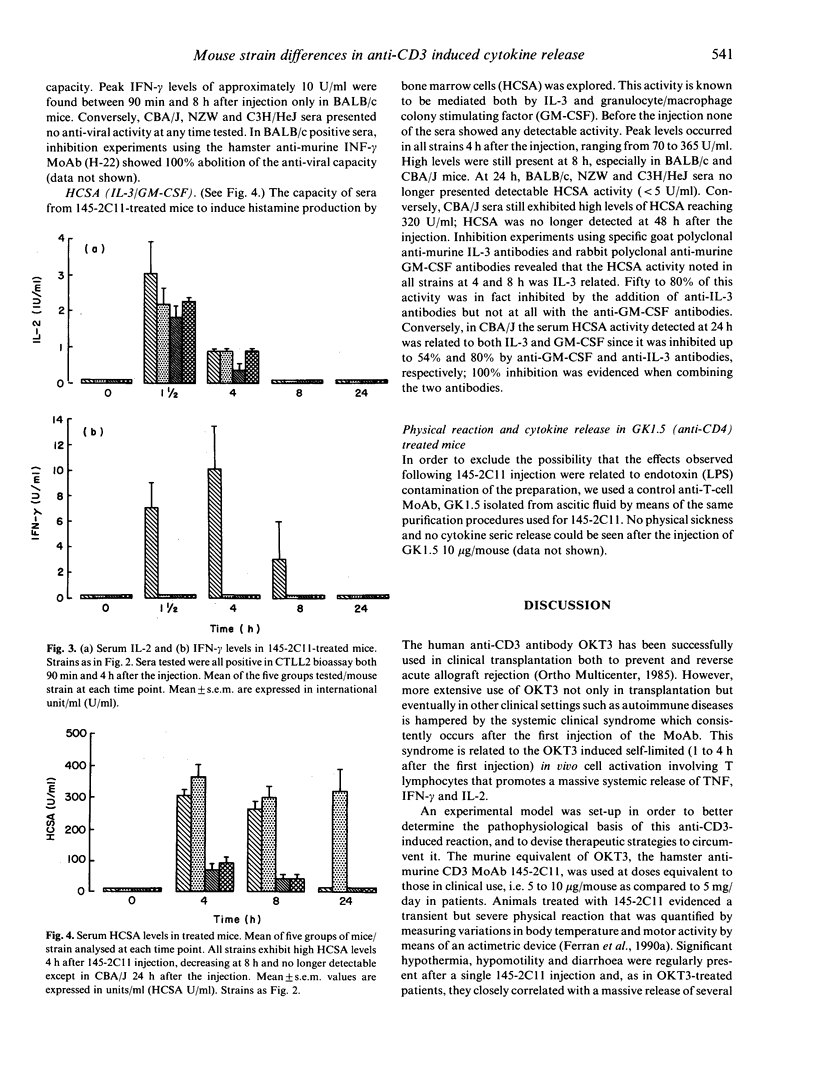
Triggering of the CD3 molecule by in vivo injection of the hamster anti-murine CD3 monoclonal antibody 145-2C11 in adult BALB/c mice leads to massive although transient T cell activation. High levels of tumour necrosis factor (TNF), interferon-gamma (IFN-gamma), IL-2, IL-3 and IL-6 are released into the circulation 1 to 8 h after a single 10 micrograms 145-2C11 i.v. injection. This release induces an impressive self-limited physical reaction associating hypothermia, hypomotility (as assessed by actimetry), diarrhoea, piloerection and even death when high doses (a single dose of greater than 100 micrograms/mouse injection) are administered. In vivo injection of 145-2C11 to other selected mouse strains, namely NZW, CBA/J and C3H/HeJ, induced both different cytokine release patterns and sickness. 145-2C11 induced significant release of TNF and IL-2 in all four strains. At variance, IFN-gamma was only detected in BALB/c mice sera which, in terms of physical reaction (hypothermia and hypomotility) were the most affected. Higher and long-lasting circulating IL-3/GM-CSF levels were present in CBA/J sera, correlating with a later recovery. These results underline heterogeneity in the in vivo cell activation pattern among different mouse strains, when triggering T lymphocytes via the CD3/Ti molecule as compared to exclusive targeting of monocyte/macrophages by means of lipopolysaccharide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Eessalu T. E., Hass P. E. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985 Dec 19;318(6047):665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Alegre M., Vandenabeele P., Flamand V., Moser M., Leo O., Abramowicz D., Urbain J., Fiers W., Goldman M. Hypothermia and hypoglycemia induced by anti-CD3 monoclonal antibody in mice: role of tumor necrosis factor. Eur J Immunol. 1990 Mar;20(3):707–710. doi: 10.1002/eji.1830200337. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Segal G. M., Harlan J. M., Adamson J. W. Tumor necrosis factor type alpha stimulates human endothelial cells to produce granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7467–7471. doi: 10.1073/pnas.83.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenoud L., Ferran C., Reuter A., Legendre C., Gevaert Y., Kreis H., Franchimont P., Bach J. F. Systemic reaction to the anti-T-cell monoclonal antibody OKT3 in relation to serum levels of tumor necrosis factor and interferon-gamma [corrected]. N Engl J Med. 1989 May 25;320(21):1420–1421. doi: 10.1056/NEJM198905253202117. [DOI] [PubMed] [Google Scholar]
- Croll A. D., Morris A. G. The regulation of gamma-interferon production by interleukins 1 and 2. Cell Immunol. 1986 Oct 1;102(1):33–42. doi: 10.1016/0008-8749(86)90323-0. [DOI] [PubMed] [Google Scholar]
- Debets J. M., Van der Linden C. J., Dieteren I. E., Leeuwenberg J. F., Buurman W. A. Fc-receptor cross-linking induces rapid secretion of tumor necrosis factor (cachectin) by human peripheral blood monocytes. J Immunol. 1988 Aug 15;141(4):1197–1201. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Ferran C., Dy M., Merite S., Sheehan K., Schreiber R., Leboulenger F., Landais P., Bluestone J., Bach J. F., Chatenoud L. Reduction of morbidity and cytokine release in anti-CD3 MoAb-treated mice by corticosteroids. Transplantation. 1990 Oct;50(4):642–648. doi: 10.1097/00007890-199010000-00023. [DOI] [PubMed] [Google Scholar]
- Ferran C., Sheehan K., Dy M., Schreiber R., Merite S., Landais P., Noel L. H., Grau G., Bluestone J., Bach J. F. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol. 1990 Mar;20(3):509–515. doi: 10.1002/eji.1830200308. [DOI] [PubMed] [Google Scholar]
- Ferran C., Sheehan K., Schreiber R., Bach J. F., Chatenoud L. Anti-TNF abrogates the cytokine-related anti-CD3 induced syndrome. Transplant Proc. 1991 Feb;23(1 Pt 1):849–850. [PubMed] [Google Scholar]
- Glode L. M., Scher I., Osborne B., Rosenstreich D. L. Cellular mechanism of endotoxin unresponsiveness in C3H/HeJ mice. J Immunol. 1976 Feb;116(2):454–461. [PubMed] [Google Scholar]
- Hirsch R., Bluestone J. A., Bare C. V., Gress R. E. Advantages of F(ab')2 fragments of anti-CD3 monoclonal antibody as compared to whole antibody as immunosuppressive agents in mice. Transplant Proc. 1991 Feb;23(1 Pt 1):270–271. [PubMed] [Google Scholar]
- Hirsch R., Eckhaus M., Auchincloss H., Jr, Sachs D. H., Bluestone J. A. Effects of in vivo administration of anti-T3 monoclonal antibody on T cell function in mice. I. Immunosuppression of transplantation responses. J Immunol. 1988 Jun 1;140(11):3766–3772. [PubMed] [Google Scholar]
- Hirsch R., Gress R. E., Pluznik D. H., Eckhaus M., Bluestone J. A. Effects of in vivo administration of anti-CD3 monoclonal antibody on T cell function in mice. II. In vivo activation of T cells. J Immunol. 1989 Feb 1;142(3):737–743. [PubMed] [Google Scholar]
- Jacob C. O., McDevitt H. O. Tumour necrosis factor-alpha in murine autoimmune 'lupus' nephritis. Nature. 1988 Jan 28;331(6154):356–358. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein M., Ettinghausen S. E., Rosenberg S. A. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986 Sep 1;137(5):1735–1742. [PubMed] [Google Scholar]
- Scott D. E., Gause W. C., Finkelman F. D., Steinberg A. D. Anti-CD3 antibody induces rapid expression of cytokine genes in vivo. J Immunol. 1990 Oct 1;145(7):2183–2188. [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Bjorndahl J. M., Wang C. Y., Kao H. T., Fu S. M. Production of tumor necrosis factor/cachectin by human T cell lines and peripheral blood T lymphocytes stimulated by phorbol myristate acetate and anti-CD3 antibody. J Exp Med. 1988 Mar 1;167(3):937–953. doi: 10.1084/jem.167.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge J. E., Bowersox O., Tribble H., Lee S. H., Shepard H. M., Liggitt D. Toxicity of tumor necrosis factor is synergistic with gamma-interferon and can be reduced with cyclooxygenase inhibitors. Am J Pathol. 1987 Sep;128(3):410–425. [PMC free article] [PubMed] [Google Scholar]
- Watson J., Largen M., McAdam K. P. Genetic control of endotoxic responses in mice. J Exp Med. 1978 Jan 1;147(1):39–49. doi: 10.1084/jem.147.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]


