Abstract
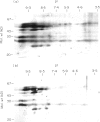
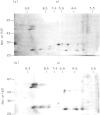
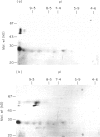
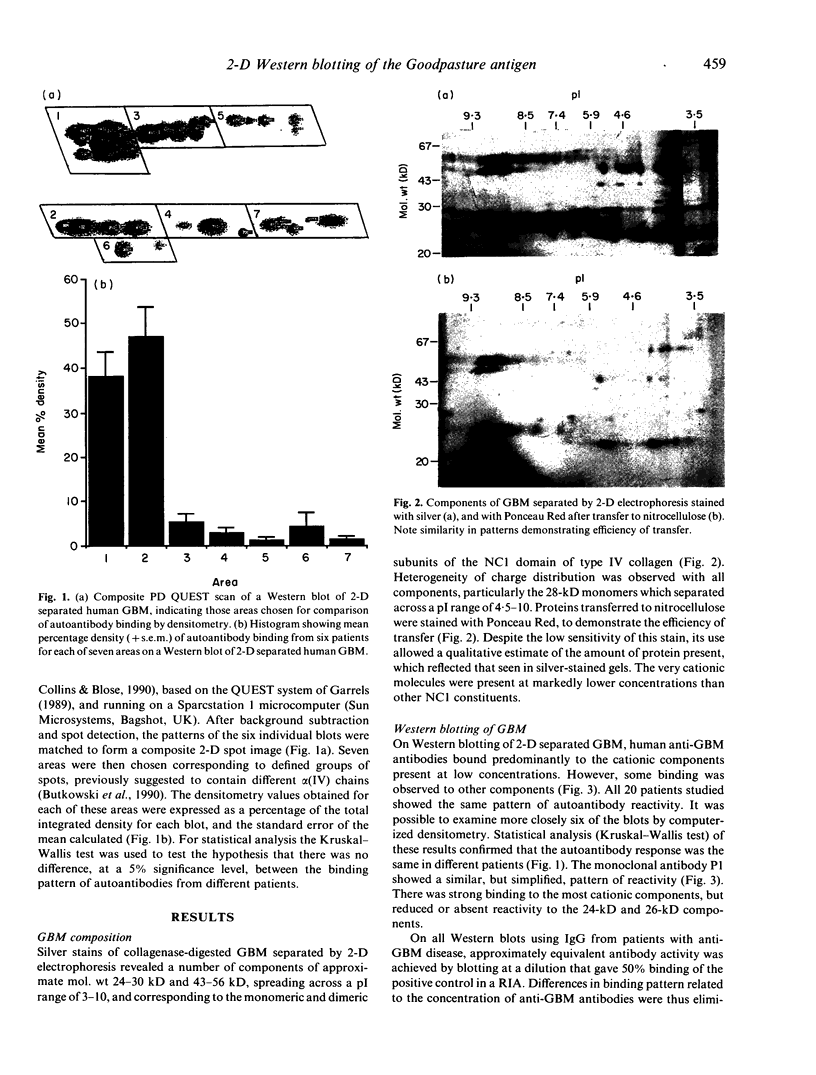
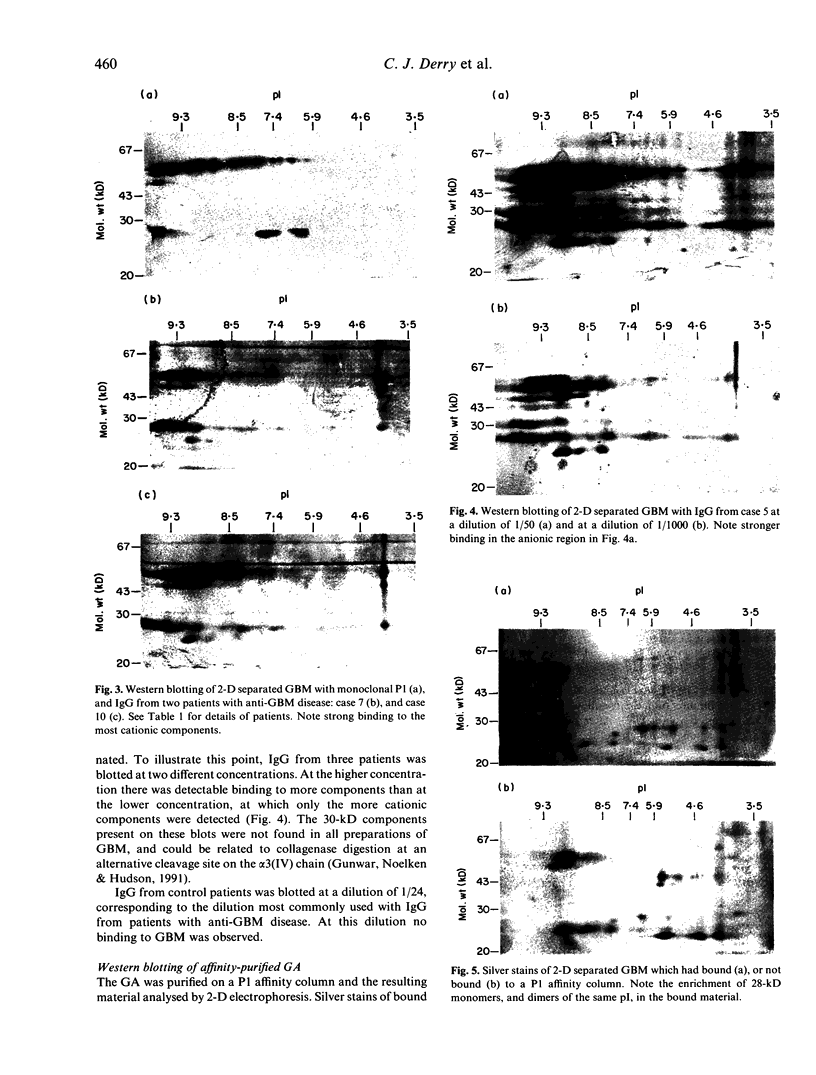
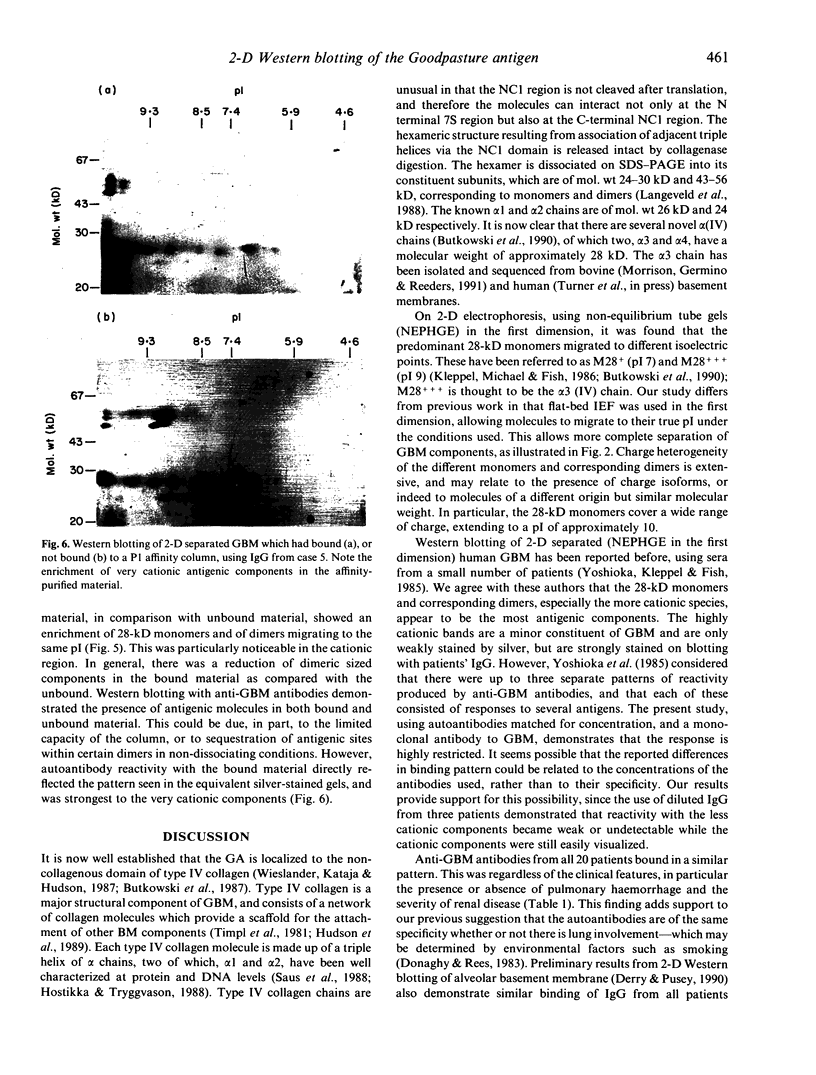
The autoantigen in Goodpasture's syndrome is known to be contained within the non-collagenous (NC1) domain of type IV collagen. We have examined the specificity of autoantibodies to glomerular basement membrane (GBM) using the technique of 2-D electrophoresis followed by Western blotting. Protein stains of 2-D gels of collagenase-digested human GBM revealed extensive charge and size heterogeneity. Major components were of mol. wt 24-30 kD and 43-56 kD, corresponding to monomeric and dimeric subunits of NCl. Western blotting of 2-D gels with IgG from patients with anti-GBM disease demonstrated that the most antigenic components migrated as cationic 28-kD monomers (pI 10) and similarly charged dimers, although other components were recognized less strongly. The mobility of the strongly antigenic polypeptides was different to that of the known alpha 1 and alpha 2 chains of type IV collagen. Autoantibodies from all 20 patients studied showed the same pattern of reactivity, regardless of their clinical features (in particular, the presence or absence of pulmonary haemorrhage) or HLA type. A monoclonal antibody (P1) to human GBM bound in a similar pattern, particularly recognizing the cationic components. 2-D gels of affinity-purified GBM from a P1 column showed enrichment of the 28-kD monomers, which were recognized by human autoantibodies on Western blotting. These results demonstrate that the autoimmune response in Goodpasture's syndrome is of restricted specificity, and support the suggestion that the major autoantigenic determinant is present on the novel alpha 3 chain of type IV collagen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D. F., Hostikka S. L., Zhou J., Chow L. T., Oliphant A. R., Gerken S. C., Gregory M. C., Skolnick M. H., Atkin C. L., Tryggvason K. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990 Jun 8;248(4960):1224–1227. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- Bowman C., Lockwood C. M. Clinical application of a radio-immunoassay for auto-antibodies to glomerular basement membrane. J Clin Lab Immunol. 1985 Aug;17(4):197–202. [PubMed] [Google Scholar]
- Butkowski R. J., Langeveld J. P., Wieslander J., Hamilton J., Hudson B. G. Localization of the Goodpasture epitope to a novel chain of basement membrane collagen. J Biol Chem. 1987 Jun 5;262(16):7874–7877. [PubMed] [Google Scholar]
- Butkowski R. J., Shen G. Q., Wieslander J., Michael A. F., Fish A. J. Characterization of type IV collagen NC1 monomers and Goodpasture antigen in human renal basement membranes. J Lab Clin Med. 1990 Mar;115(3):365–373. [PubMed] [Google Scholar]
- Cashman S. J., Pusey C. D., Evans D. J. Extraglomerular distribution of immunoreactive Goodpasture antigen. J Pathol. 1988 May;155(1):61–70. doi: 10.1002/path.1711550110. [DOI] [PubMed] [Google Scholar]
- Donaghy M., Rees A. J. Cigarette smoking and lung haemorrhage in glomerulonephritis caused by autoantibodies to glomerular basement membrane. Lancet. 1983 Dec 17;2(8364):1390–1393. doi: 10.1016/s0140-6736(83)90923-6. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. The QUEST system for quantitative analysis of two-dimensional gels. J Biol Chem. 1989 Mar 25;264(9):5269–5282. [PubMed] [Google Scholar]
- Gunwar S., Noelken M. E., Hudson B. G. Properties of the collagenous domain of the alpha 3(IV) chain, the Goodpasture antigen, of lens basement membrane collagen. Selective cleavage of alpha (IV) chains with retention of their triple helical structure and noncollagenous domain. J Biol Chem. 1991 Jul 25;266(21):14088–14094. [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Hostikka S. L., Eddy R. L., Byers M. G., Höyhtyä M., Shows T. B., Tryggvason K. Identification of a distinct type IV collagen alpha chain with restricted kidney distribution and assignment of its gene to the locus of X chromosome-linked Alport syndrome. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1606–1610. doi: 10.1073/pnas.87.4.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostikka S. L., Tryggvason K. The complete primary structure of the alpha 2 chain of human type IV collagen and comparison with the alpha 1(IV) chain. J Biol Chem. 1988 Dec 25;263(36):19488–19493. [PubMed] [Google Scholar]
- Hudson B. G., Wieslander J., Wisdom B. J., Jr, Noelken M. E. Goodpasture syndrome: molecular architecture and function of basement membrane antigen. Lab Invest. 1989 Sep;61(3):256–269. [PubMed] [Google Scholar]
- Kleppel M. M., Michael A. F., Fish A. J. Antibody specificity of human glomerular basement membrane type IV collagen NC1 subunits. Species variation in subunit composition. J Biol Chem. 1986 Dec 15;261(35):16547–16552. [PubMed] [Google Scholar]
- Krauss M. R., Collins P. J., Blose S. H. A two-dimensional acrylamide gel electrophoresis/computer software approach to decoding the human genome. Biotechniques. 1990 Feb;8(2):218–223. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langeveld J. P., Wieslander J., Timoneda J., McKinney P., Butkowski R. J., Wisdom B. J., Jr, Hudson B. G. Structural heterogeneity of the noncollagenous domain of basement membrane collagen. J Biol Chem. 1988 Jul 25;263(21):10481–10488. [PubMed] [Google Scholar]
- Lerner R. A., Glassock R. J., Dixon F. J. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967 Dec 1;126(6):989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison K. E., Germino G. G., Reeders S. T. Use of the polymerase chain reaction to clone and sequence a cDNA encoding the bovine alpha 3 chain of type IV collagen. J Biol Chem. 1991 Jan 5;266(1):34–39. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Pusey C. D., Dash A., Kershaw M. J., Morgan A., Reilly A., Rees A. J., Lockwood C. M. A single autoantigen in Goodpasture's syndrome identified by a monoclonal antibody to human glomerular basement membrane. Lab Invest. 1987 Jan;56(1):23–31. [PubMed] [Google Scholar]
- Pusey C. D., Lockwood C. M., Peters D. K. Plasma exchange and immunosuppressive drugs in the treatment of glomerulonephritis due to antibodies to the glomerular basement membrane. Int J Artif Organs. 1983 Jul;6 (Suppl 1):15–18. [PubMed] [Google Scholar]
- Saus J., Wieslander J., Langeveld J. P., Quinones S., Hudson B. G. Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J Biol Chem. 1988 Sep 15;263(26):13374–13380. [PubMed] [Google Scholar]
- Savage C. O., Pusey C. D., Kershaw M. J., Cashman S. J., Harrison P., Hartley B., Turner D. R., Cameron J. S., Evans D. J., Lockwood C. M. The Goodpasture antigen in Alport's syndrome: studies with a monoclonal antibody. Kidney Int. 1986 Jul;30(1):107–112. doi: 10.1038/ki.1986.158. [DOI] [PubMed] [Google Scholar]
- Shu S. Y., Ju G., Fan L. Z. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988 Feb 29;85(2):169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981 Nov;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander J., Barr J. F., Butkowski R. J., Edwards S. J., Bygren P., Heinegård D., Hudson B. G. Goodpasture antigen of the glomerular basement membrane: localization to noncollagenous regions of type IV collagen. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3838–3842. doi: 10.1073/pnas.81.12.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander J., Kataja M., Hudson B. G. Characterization of the human Goodpasture antigen. Clin Exp Immunol. 1987 Aug;69(2):332–340. [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K., Kleppel M., Fish A. J. Analysis of nephritogenic antigens in human glomerular basement membrane by two-dimensional gel electrophoresis. J Immunol. 1985 Jun;134(6):3831–3837. [PubMed] [Google Scholar]