Abstract
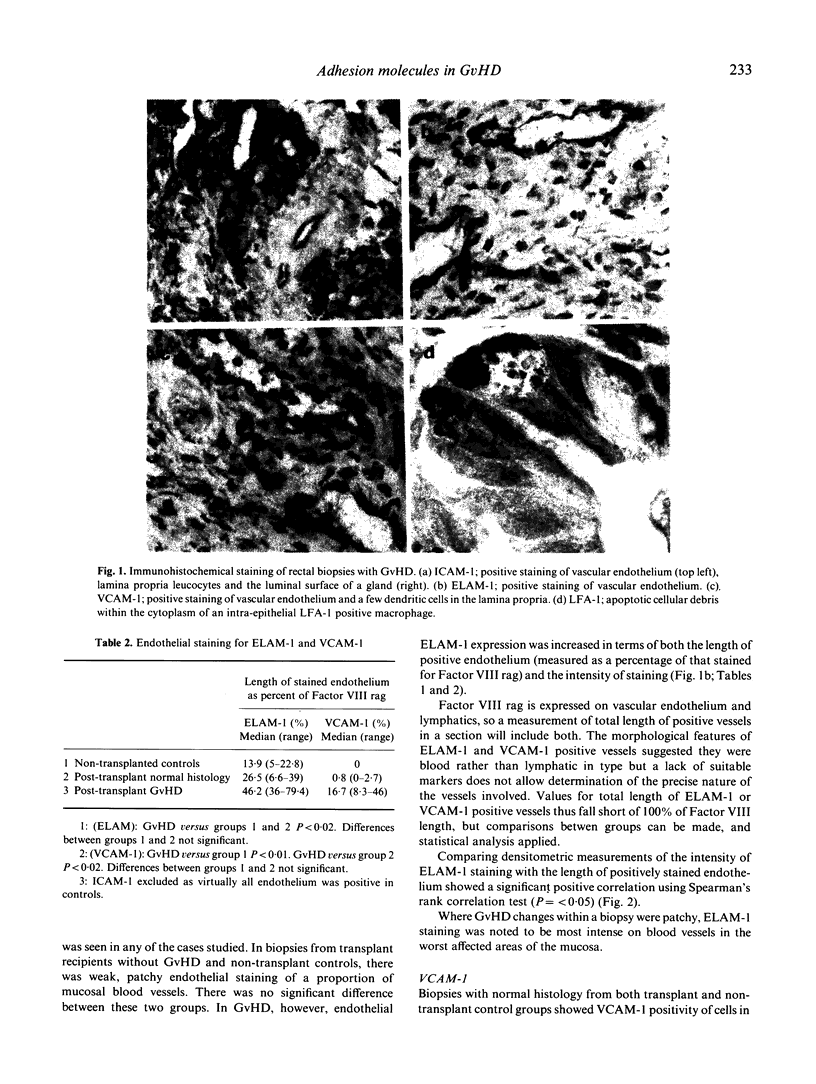
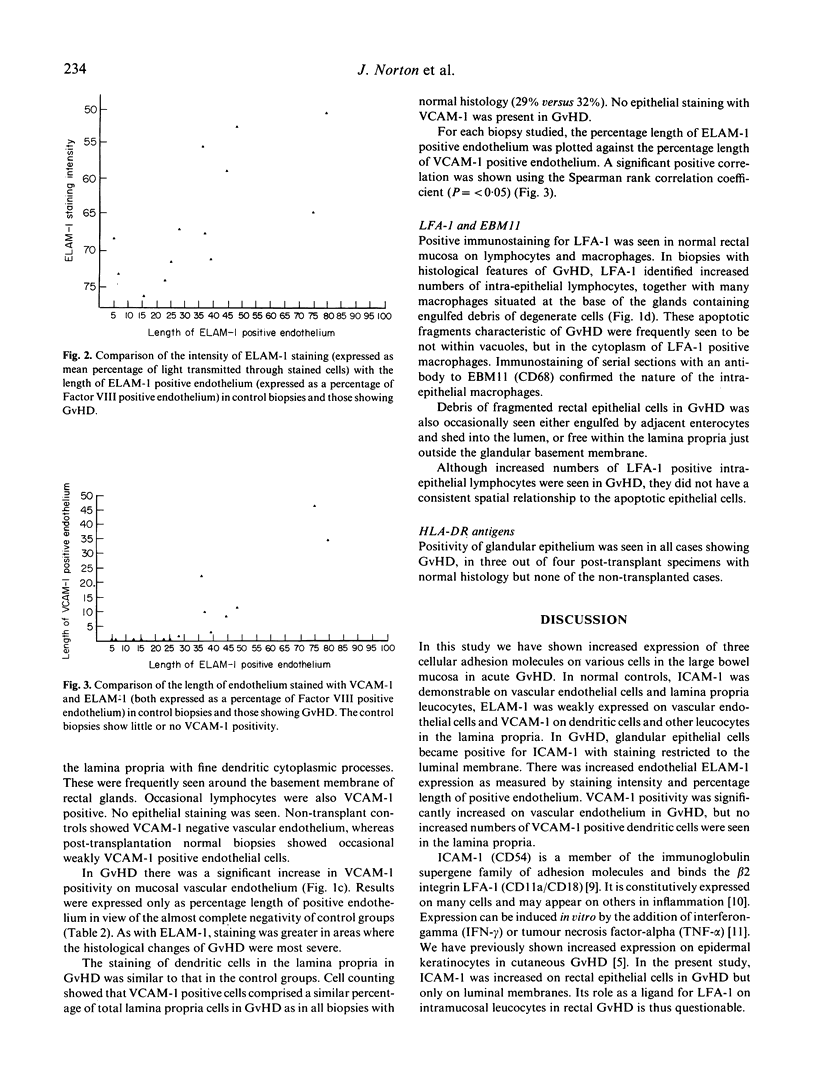
The distribution of three cellular adhesion molecules, ICAM-1, ELAM-1 and VCAM-1, was studied in normal rectal mucosa and in graft-versus-host disease (GvHD) using immunohistological and morphometric techniques. In normal controls, ICAM-1 was demonstrable on endothelial cells and leucocytes within the lamina propria, ELAM-1 on endothelial cells only and VCAM-1 on lamina propria leucocytes, many of which exhibited long dendritic processes surrounding the glands. In GvHD, the enterocytes became positive for ICAM-1 and this was often associated with the presence of intra-epithelial LFA-1+ lymphocytes and macrophages, the latter containing debris of apoptotic cells. The staining was, however, restricted to the luminal membrane of the epithelial cells, raising doubts about the role of ICAM-1 as a ligand for LFA-1 on mucosal leucocytes in rectal GvHD. ELAM-1 expression was increased in GvHD both in terms of the length of positive endothelium and staining intensity. VACM-1 was increased on endothelial cells but not leucocytes in the lamina propria in contrast to our previous findings in cutaneous GvHD where VCAM-1+ dendritic cells were increased and endothelial cells remained negative. Normal patterns of adhesion molecule staining were seen in two biopsies exhibiting no morphological evidence of GvHD, from patients who had strong clinical evidence of the disease, indicating that immunostaining for these molecules is unlikely to be of help in improving the sensitivity of histological diagnosis. However, the possibility that adhesion molecule staining may be useful in improving diagnostic specificity by helping to distinguish GvHD from identical histological changes produced by irradiation and cytotoxic drugs is worthy of further investigation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briscoe D. M., Schoen F. J., Rice G. E., Bevilacqua M. P., Ganz P., Pober J. S. Induced expression of endothelial-leukocyte adhesion molecules in human cardiac allografts. Transplantation. 1991 Feb;51(2):537–539. [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilly S. A., Sloane J. P. Changes in rectal leucocytes after allogeneic bone marrow transplantation. Clin Exp Immunol. 1987 Jan;67(1):151–158. [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Epstein R. J., McDonald G. B., Sale G. E., Shulman H. M., Thomas E. D. The diagnostic accuracy of the rectal biopsy in acute graft-versus-host disease: a prospective study of thirteen patients. Gastroenterology. 1980 Apr;78(4):764–771. [PubMed] [Google Scholar]
- Griffiths C. E., Voorhees J. J., Nickoloff B. J. Characterization of intercellular adhesion molecule-1 and HLA-DR expression in normal and inflamed skin: modulation by recombinant gamma interferon and tumor necrosis factor. J Am Acad Dermatol. 1989 Apr;20(4):617–629. doi: 10.1016/s0190-9622(89)70073-6. [DOI] [PubMed] [Google Scholar]
- Lerner K. G., Kao G. F., Storb R., Buckner C. D., Clift R. A., Thomas E. D. Histopathology of graft-vs.-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplant Proc. 1974 Dec;6(4):367–371. [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Norton J., Sloane J. P. ICAM-1 expression on epidermal keratinocytes in cutaneous graft-versus-host disease. Transplantation. 1991 Jun;51(6):1203–1206. doi: 10.1097/00007890-199106000-00011. [DOI] [PubMed] [Google Scholar]
- Norton J., Sloane J. P., al-Saffar N., Haskard D. O. Vessel associated adhesion molecules in normal skin and acute graft-versus-host disease. J Clin Pathol. 1991 Jul;44(7):586–591. doi: 10.1136/jcp.44.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Corless C., Bevilacqua M. P. Vascular and nonvascular expression of INCAM-110. A target for mononuclear leukocyte adhesion in normal and inflamed human tissues. Am J Pathol. 1991 Feb;138(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Snover D. C. Biopsy interpretation in bone marrow transplantation. Pathol Annu. 1989;24(Pt 2):63–101. [PubMed] [Google Scholar]
- Snover D. C. Mucosal damage simulating acute graft-versus-host reaction in cytomegalovirus colitis. Transplantation. 1985 Jun;39(6):669–670. [PubMed] [Google Scholar]
- Sviland L., Pearson A. D., Eastham E. J., Green M. A., Hamilton P. J., Proctor S. J., Malcolm A. J. Class II antigen expression by keratinocytes and enterocytes--an early feature of graft-versus-host-disease. Transplantation. 1988 Sep;46(3):402–406. doi: 10.1097/00007890-198809000-00014. [DOI] [PubMed] [Google Scholar]
- Wellicome S. M., Thornhill M. H., Pitzalis C., Thomas D. S., Lanchbury J. S., Panayi G. S., Haskard D. O. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990 Apr 1;144(7):2558–2565. [PubMed] [Google Scholar]



