Abstract
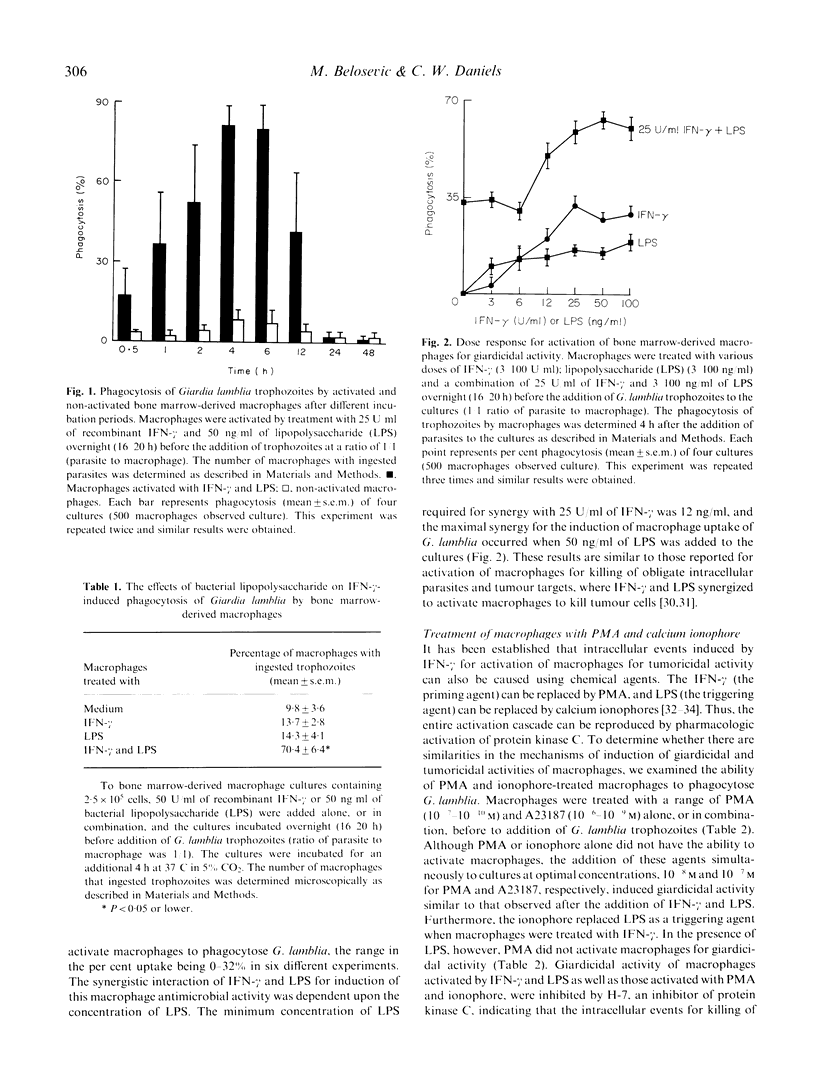
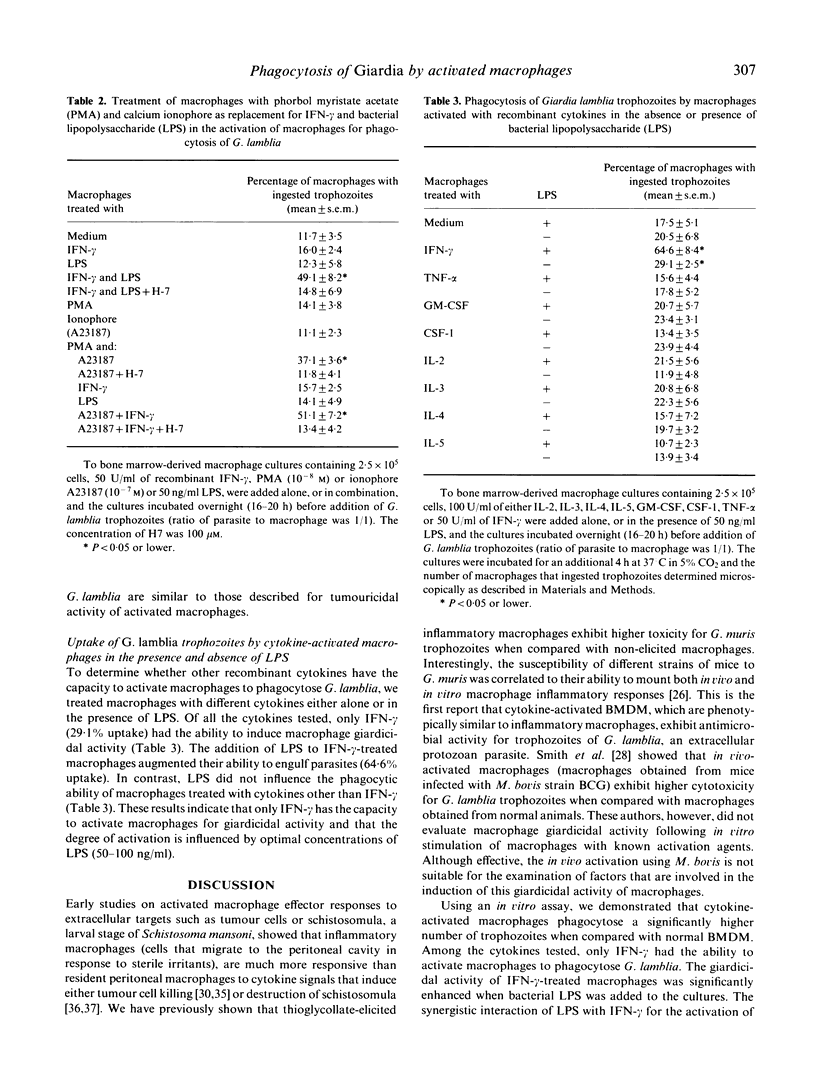
Phagocytosis of Giardia lamblia trophozoites by cytokine-activated and non-activated bone marrow-derived macrophages was examined in vitro. Macrophages treated with recombinant interferon-gamma (IFN-gamma) and bacterial lipopolysaccharide (LPS) ingested a significantly higher number of in vitro-grown trophozoites than untreated macrophages. Maximal uptake of parasites occurred after 4 h and 6 h of incubation where 81.4% and 79.1% of macrophages were positive for trophozoites. Other cytokines tested, IL-2, IL-3, IL-4, IL-5, GM-CSF, CSF-1 and tumour necrosis factor-alpha (TNF-alpha) either alone or in combination with LPS, failed to activate macrophages to phagocytose G. lamblia. The induction of this activated macrophage anti-microbial function was achieved pharmacologically using phorbol myristate acetate (PMA) and ionophore A23187. The giardicidal activity of macrophages activated with IFN-gamma and LPS or that induced by PMA and A23187 was inhibited by H-7, indicating the role for protein kinase C in the intracellular events following activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Roberts-Thomson I. C., Mitchell G. F. Giardiasis in mice: analysis of humoral and cellular immune responses to Giardia muris. Parasite Immunol. 1982 Jan;4(1):47–57. doi: 10.1111/j.1365-3024.1982.tb00419.x. [DOI] [PubMed] [Google Scholar]
- Andrews J. S., Jr, Hewlett E. L. Protection against infection with Giardia muris by milk containing antibody to Giardia. J Infect Dis. 1981 Feb;143(2):242–246. doi: 10.1093/infdis/143.2.242. [DOI] [PubMed] [Google Scholar]
- Becton D. L., Adams D. O., Hamilton T. A. Characterization of protein kinase C activity in interferon gamma treated murine peritoneal macrophages. J Cell Physiol. 1985 Dec;125(3):485–491. doi: 10.1002/jcp.1041250318. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988 Aug 1;141(3):890–896. [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M. Comparative studies of inflammatory responses in susceptible and resistant mice infected with Giardia muris. Clin Exp Immunol. 1986 Sep;65(3):622–630. [PMC free article] [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M. Killing of Giardia muris trophozoites in vitro by spleen, mesenteric lymph node and peritoneal cells from susceptible and resistant mice. Immunology. 1986 Oct;59(2):269–275. [PMC free article] [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M. Lysis and immobilization of Giardia muris trophozoites in vitro by immune serum from susceptible and resistant mice. Parasite Immunol. 1987 Jan;9(1):11–19. doi: 10.1111/j.1365-3024.1987.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M., MacLean J. D., Law C., Croll N. A. Giardia lamblia infections in Mongolian gerbils: an animal model. J Infect Dis. 1983 Feb;147(2):222–226. doi: 10.1093/infdis/147.2.222. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M., MacLean J. D. The effects of cyclosporin A on the course of infection with Giardia muris in mice. Am J Trop Med Hyg. 1986 May;35(3):496–500. doi: 10.4269/ajtmh.1986.35.496. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M. Temporal study of acquired resistance in infections of mice with Giardia muris. Parasitology. 1983 Dec;87(Pt 3):517–524. doi: 10.1017/s0031182000083037. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Meltzer M. S., Nacy C. A. IL-2. A cofactor for induction of activated macrophage resistance to infection. J Immunol. 1990 Aug 1;145(3):831–839. [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Mahmoud A. A. Killing of schistosomula of Schistosoma mansoni by normal human monocytes. J Immunol. 1979 Aug;123(2):949–951. [PubMed] [Google Scholar]
- Erlich J. H., Anders R. F., Roberts-Thomson I. C., Schrader J. W., Mitchell G. F. An examination of differences in serum antibody specificities and hypersensitivity reactions as contributing factors to chronic infection with the intestinal protozoan parasite, Giardia muris, in mice. Aust J Exp Biol Med Sci. 1983 Oct;61(Pt 5):599–615. doi: 10.1038/icb.1983.57. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Becton D. L., Somers S. D., Gray P. W., Adams D. O. Interferon-gamma modulates protein kinase C activity in murine peritoneal macrophages. J Biol Chem. 1985 Feb 10;260(3):1378–1381. [PubMed] [Google Scholar]
- Heyworth M. F. Antibody response to Giardia muris trophozoites in mouse intestine. Infect Immun. 1986 May;52(2):568–571. doi: 10.1128/iai.52.2.568-571.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. R., Pearson R. D. Ingestion of Giardia lamblia trophozoites by human mononuclear phagocytes. Infect Immun. 1987 Dec;55(12):3155–3161. doi: 10.1128/iai.55.12.3155-3161.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. R., Pohl R. Ingestion of Giardia lamblia trophozoites by murine Peyer's patch macrophages. Infect Immun. 1990 Oct;58(10):3202–3207. doi: 10.1128/iai.58.10.3202-3207.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istre G. R., Dunlop T. S., Gaspard G. B., Hopkins R. S. Waterborne giardiasis at a mountain resort: evidence for acquired immunity. Am J Public Health. 1984 Jun;74(6):602–604. doi: 10.2105/ajph.74.6.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L., Leonard E. J., Meltzer M. S. Macrophages as effector cells of protective immunity in murine schistosomiasis. IV. Coincident induction of macrophage activation for extracellular killing of schistosomula and tumor cells. Cell Immunol. 1982 Nov 15;74(1):86–96. doi: 10.1016/0008-8749(82)90008-9. [DOI] [PubMed] [Google Scholar]
- James S. L., Sher A., Lazdins J. K., Meltzer M. S. Macrophages as effector cells of protective immunity in murine schistosomiasis. II. Killing of newly transformed schistosomula in vitro by macrophages activated as a consequence of Schistosoma mansoni infection. J Immunol. 1982 Apr;128(4):1535–1540. [PubMed] [Google Scholar]
- Janoff E. N., Smith P. D., Blaser M. J. Acute antibody responses to Giardia lamblia are depressed in patients with AIDS. J Infect Dis. 1988 Apr;157(4):798–804. doi: 10.1093/infdis/157.4.798. [DOI] [PubMed] [Google Scholar]
- Kaplan B. S., Uni S., Aikawa M., Mahmoud A. A. Effector mechanism of host resistance in murine giardiasis: specific IgG and IgA cell-mediated toxicity. J Immunol. 1985 Mar;134(3):1975–1981. [PubMed] [Google Scholar]
- Kassis A. I., Aikawa M., Mahmoud A. F. Mouse antibody-dependent eosinophil and macrophage adherence and damage to schistosomula of Schistosoma mansoni. J Immunol. 1979 Feb;122(2):398–405. [PubMed] [Google Scholar]
- Kongshavn P. A., Sadarangani C., Skamene E. Genetically determined differences in antibacterial activity of macrophages are expressed in the environment in which the macrophage precursors mature. Cell Immunol. 1980 Aug 1;53(2):341–349. doi: 10.1016/0008-8749(80)90334-2. [DOI] [PubMed] [Google Scholar]
- Lewis P. D., Jr, Belosevic M., Faubert G. M., Curthoys L., MacLean J. D. Cortisone-induced recrudescence of Giardia lamblia infections in gerbils. Am J Trop Med Hyg. 1987 Jan;36(1):33–40. doi: 10.4269/ajtmh.1987.36.33. [DOI] [PubMed] [Google Scholar]
- Lowell G. H., MacDermott R. P., Summers P. L., Reeder A. A., Bertovich M. J., Formal S. B. Antibody-dependent cell-mediated antibacterial activity: K lymphocytes, monocytes, and granulocytes are effective against shigella. J Immunol. 1980 Dec;125(6):2778–2784. [PubMed] [Google Scholar]
- Meltzer M. S. Macrophage activation for tumor cytotoxicity: characterization of priming and trigger signals during lymphokine activation. J Immunol. 1981 Jul;127(1):179–183. [PubMed] [Google Scholar]
- Moore G. T., Cross W. M., McGuire D., Mollohan C. S., Gleason N. N., Healy G. R., Newton L. H. Epidemic giardiasis at a ski resort. N Engl J Med. 1969 Aug 21;281(8):402–407. doi: 10.1056/NEJM196908212810802. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Oster C. N., James S. L., Meltzer M. S. Activation of macrophages to kill rickettsiae and Leishmania: dissociation of intracellular microbicidal activities and extracellular destruction of neoplastic and helminth targets. Contemp Top Immunobiol. 1984;13:147–170. doi: 10.1007/978-1-4757-1445-6_8. [DOI] [PubMed] [Google Scholar]
- Nair K. V., Gillon J., Ferguson A. Corticosteroid treatment increases parasite numbers in murine giardiasis. Gut. 1981 Jun;22(6):475–480. doi: 10.1136/gut.22.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. L., Allen C. L., Stevens D. P. Phagocytosis of Giardia muris by macrophages in Peyer's patch epithelium in mice. Infect Immun. 1981 Aug;33(2):591–601. doi: 10.1128/iai.33.2.591-601.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulescu S., Meyer E. A. Opsonization in vitro of Giardia lamblia trophozoites. Infect Immun. 1981 May;32(2):852–856. doi: 10.1128/iai.32.2.852-856.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Mitchell G. F. Giardiasis in mice. I. Prolonged infections in certain mouse strains and hypothymic (nude) mice. Gastroenterology. 1978 Jul;75(1):42–46. [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Mitchell G. F. Protection of mice against Giardia muris infection. Infect Immun. 1979 Jun;24(3):971–973. doi: 10.1128/iai.24.3.971-973.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: development of macrophage cytotoxic activity requires completion of a sequence of short-lived intermediary reactions. J Immunol. 1978 Nov;121(5):2035–2042. [PubMed] [Google Scholar]
- Smith P. D., Elson C. O., Keister D. B., Nash T. E. Human host response to Giardia lamblia. I. Spontaneous killing by mononuclear leukocytes in vitro. J Immunol. 1982 Mar;128(3):1372–1376. [PubMed] [Google Scholar]
- Smith P. D., Keister D. B., Wahl S. M., Meltzer M. S. Defective spontaneous but normal antibody-dependent cytotoxicity for an extracellular protozoan parasite, Giardia lamblia, by C3H/HeJ mouse macrophages. Cell Immunol. 1984 Apr 15;85(1):244–251. doi: 10.1016/0008-8749(84)90294-6. [DOI] [PubMed] [Google Scholar]
- Snider D. P., Gordon J., McDermott M. R., Underdown B. J. Chronic Giardia muris infection in anti-IgM-treated mice. I. Analysis of immunoglobulin and parasite-specific antibody in normal and immunoglobulin-deficient animals. J Immunol. 1985 Jun;134(6):4153–4162. [PubMed] [Google Scholar]
- Snider D. P., Underdown B. J. Quantitative and temporal analyses of murine antibody response in serum and gut secretions to infection with Giardia muris. Infect Immun. 1986 Apr;52(1):271–278. doi: 10.1128/iai.52.1.271-278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. P., Frank D. M., Mahmoud A. A. Thymus dependency of host resistance to Giardia muris infection: studies in nude mice. J Immunol. 1978 Feb;120(2):680–682. [PubMed] [Google Scholar]
- Stevenson M. M., Kongshavn P. A., Skamene E. Genetic linkage of resistance to Listeria monocytogenes with macrophage inflammatory responses. J Immunol. 1981 Aug;127(2):402–407. [PubMed] [Google Scholar]
- Underdown B. J., Roberts-Thomson I. C., Anders R. F., Mitchell G. F. Giardiasis in mice: studies on the characteristics of chronic infection in C3h/He mice. J Immunol. 1981 Feb;126(2):669–672. [PubMed] [Google Scholar]
- Underdown B. J., Skea D. L., Loney G. M., Snider D. P. Murine giardiasis and mucosal immunity: a model for the study of immunity to intestinal protozoan parasites. Monogr Allergy. 1988;24:287–296. [PubMed] [Google Scholar]


