Abstract
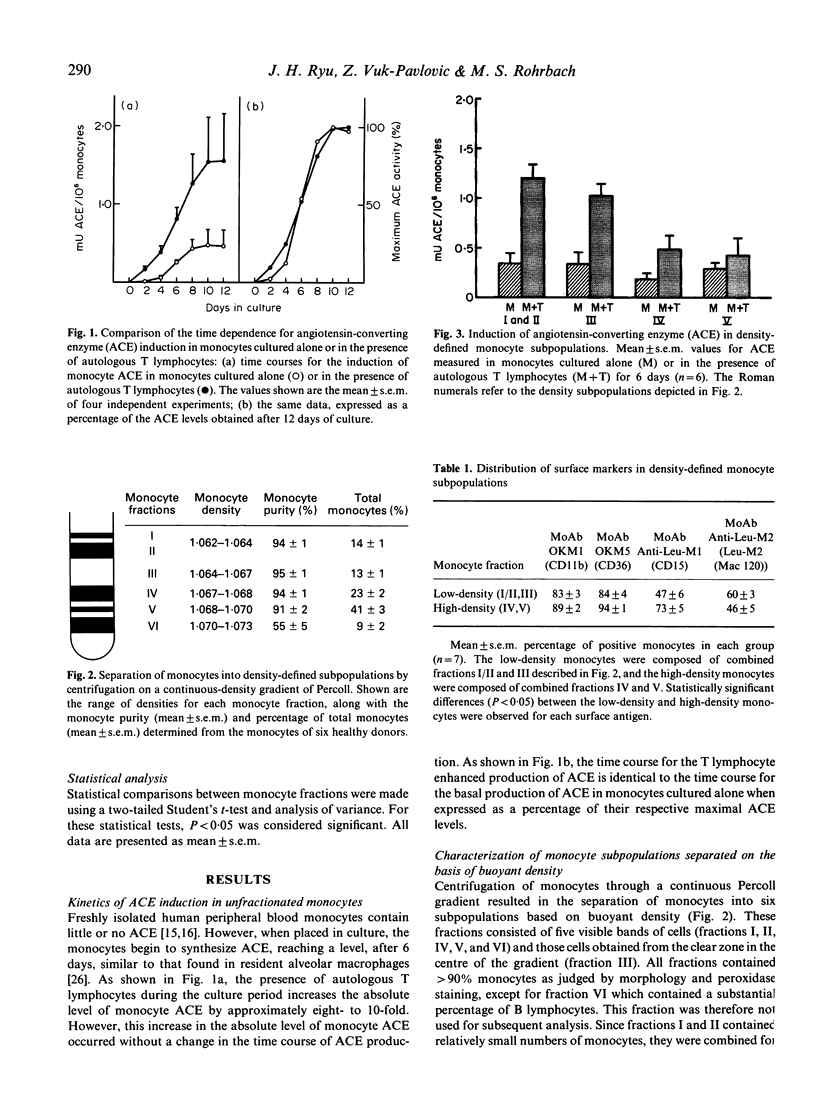
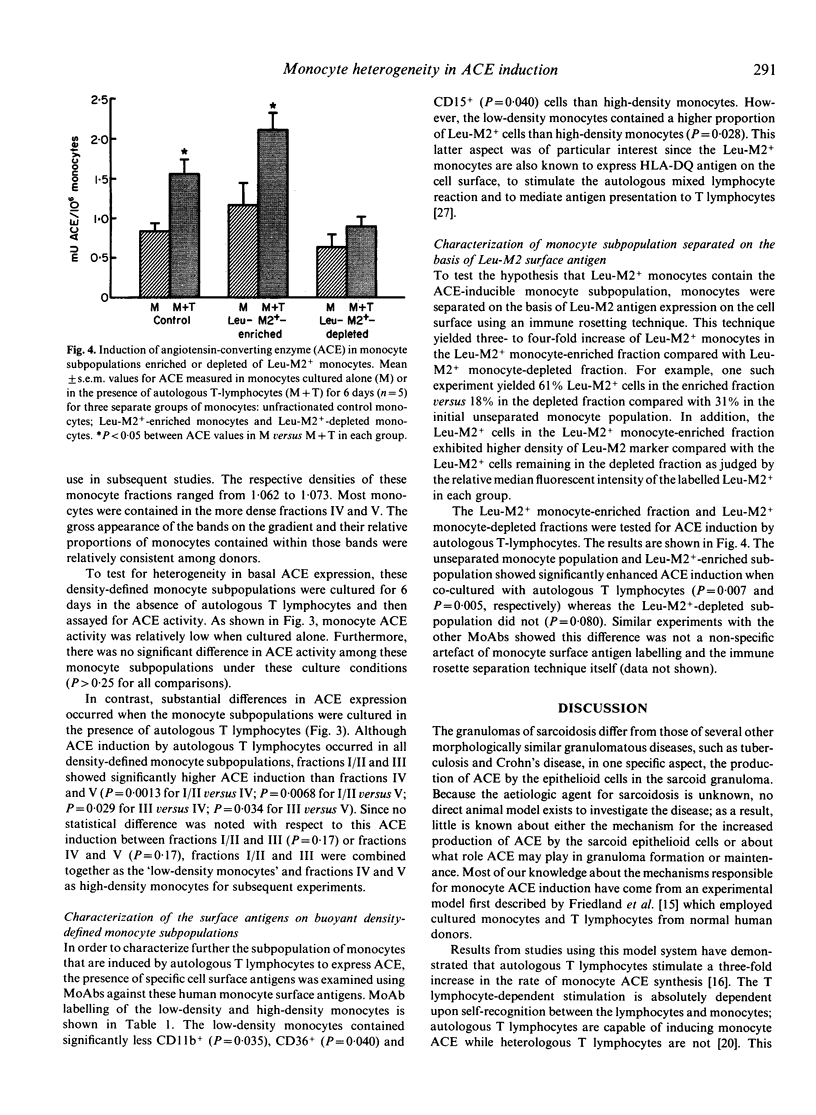
The ability of monocyte subpopulations to be induced selectively by T lymphocytes to synthesize enhanced levels of angiotensin-converting enzyme (ACE) was examined using an in vitro model employing normal peripheral blood monocytes and T lymphocytes. Separation of monocytes into subpopulations on the basis of buoyant density indicated no difference in the ability of the resulting monocyte subpopulations to produce basal levels of ACE when cultured in the absence of T lymphocytes. However, the subpopulations differed significantly in their ability to synthesize enhanced levels of ACE in response to the presence of autologous T lymphocytes; low-density monocytes were induced by T lymphocytes to synthesize three-fold more ACE than were high-density monocytes. Surface antigen labelling using MoAbs demonstrated that the low-density monocyte subpopulations also had a significantly higher percentage of Leu-M2+ monocytes compared with the high-density monocyte subpopulations. When monocytes were separated on the basis of the presence of the Leu-M2 antigen using an immune rosetting technique, T lymphocytes were able to induce significantly elevated levels of ACE in the Leu-M2+ enriched monocyte subpopulation but were unable to induce ACE beyond basal levels in the Leu-M2(+)-depleted monocyte subpopulation. These results demonstrate that monocytes are heterogeneous with respect to their ability to be induced by T lymphocytes to synthesize ACE. This raises the possibility that selective accumulation of a monocyte subpopulation in the granulomatous inflammation of sarcoidosis may be one of the factors required for elevated ACE synthesis in the resulting granuloma epithelioid cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Miller P. J., Thurman G. B., Neubauer R. H., Oliver C., Favilla T., Beman J. A., Oldham R. K., Stevenson H. C. Characterization of a human blood monocyte subset with low peroxidase activity. J Clin Invest. 1983 Sep;72(3):1093–1105. doi: 10.1172/JCI111034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad A. K., Rohrbach M. S. An in vitro model for the induction of angiotensin-converting enzyme in sarcoidosis. Evidence for a soluble ACE-inducing factor. Am Rev Respir Dis. 1987 Feb;135(2):396–400. doi: 10.1164/arrd.1987.135.2.396. [DOI] [PubMed] [Google Scholar]
- Daniele R. P., Dauber J. H., Rossman M. D. Immunologic abnormalities in sarcoidosis. Ann Intern Med. 1980 Mar;92(3):406–416. doi: 10.7326/0003-4819-92-3-406. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Ferro T. J., Rossman M. D., Greenberg J. A., Daniele R. P., Schreiber A. D., Freundlich B. Differential prostaglandin production by unfractionated and density-fractionated human monocytes and alveolar macrophages. J Leukoc Biol. 1987 Aug;42(2):114–121. doi: 10.1002/jlb.42.2.114. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Schreiber A. D., Gustilo K., Chien P., Rossman M. D., Lammie P. J., Daniele R. P. Differential interleukin 1 elaboration by unfractionated and density fractionated human alveolar macrophages and blood monocytes: relationship to cell maturity. J Immunol. 1985 Nov;135(5):3198–3204. [PubMed] [Google Scholar]
- Epstein W. L. Granuloma formation in man. Pathobiol Annu. 1977;7:1–30. [PubMed] [Google Scholar]
- Freudenthal P. S., Steinman R. M. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7698–7702. doi: 10.1073/pnas.87.19.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland J., Setton C., Silverstein E. Induction of angiotensin converting enzyme in human monocytes in culture. Biochem Biophys Res Commun. 1978 Aug 14;83(3):843–849. doi: 10.1016/0006-291x(78)91471-7. [DOI] [PubMed] [Google Scholar]
- Gmelig-Meyling F., Waldmann T. A. Separation of human blood monocytes and lymphocytes on a continuous Percoll gradient. J Immunol Methods. 1980;33(1):1–9. doi: 10.1016/0022-1759(80)90077-0. [DOI] [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Goldyne M. E., Stobo J. D. Synthesis of prostaglandins by subpopulations of human peripheral blood monocytes. Prostaglandins. 1979 Nov;18(5):687–695. doi: 10.1016/0090-6980(79)90089-3. [DOI] [PubMed] [Google Scholar]
- Gonwa T. A., Picker L. J., Raff H. V., Goyert S. M., Silver J., Stobo J. D. Antigen-presenting capabilities of human monocytes correlates with their expression of HLA-DS, an Ia determinant distinct from HLA-DR. J Immunol. 1983 Feb;130(2):706–711. [PubMed] [Google Scholar]
- Gonwa T. A., Stobo J. D. Differential expression of Ia molecules by human monocytes. J Clin Invest. 1984 Sep;74(3):859–866. doi: 10.1172/JCI111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. D., Beveridge R. P., Schlossman S. F. Isolation of myeloid progenitor cells from peripheral blood of chronic myelogenous leukemia patients. Blood. 1982 Jul;60(1):30–37. [PubMed] [Google Scholar]
- Haslam P. L., Parker D. J., Townsend P. J. Increases in HLA-DQ, DP, DR, and transferrin receptors on alveolar macrophages in sarcoidosis and allergic alveolitis compared with fibrosing alveolitis. Chest. 1990 Mar;97(3):651–661. doi: 10.1378/chest.97.3.651. [DOI] [PubMed] [Google Scholar]
- Hausman P. B., Raff H. V., Gilbert R. C., Picker L. J., Stobo J. D. T cells and macrophages involved in the autologous mixed lymphocyte reaction are required for the response to conventional antigen. J Immunol. 1980 Sep;125(3):1374–1379. [PubMed] [Google Scholar]
- Hinman L. M., Stevens C., Matthay R. A., Bernard J., Gee L. Angiotensin convertase activities in human alveolar macrophages: effects of cigarette smoking and sarcoidosis. Science. 1979 Jul 13;205(4402):202–203. doi: 10.1126/science.221980. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Young R. C., Jr, Kawanami O., Ferrans V. J., Crystal R. G. Maintenance of granuloma formation in pulmonary sarcoidosis by T lymphocytes within the lung. N Engl J Med. 1980 Mar 13;302(11):594–598. doi: 10.1056/NEJM198003133021102. [DOI] [PubMed] [Google Scholar]
- James D. G., Williams W. J. Immunology of sarcoidosis. Am J Med. 1982 Jan;72(1):5–8. doi: 10.1016/0002-9343(82)90564-2. [DOI] [PubMed] [Google Scholar]
- Khansari N., Chou Y. K., Fudenberg H. H. Human monocyte heterogeneity: interleukin 1 and prostaglandin E2 production by separate subsets. Eur J Immunol. 1985 Jan;15(1):48–51. doi: 10.1002/eji.1830150110. [DOI] [PubMed] [Google Scholar]
- Lasser A. The mononuclear phagocytic system: a review. Hum Pathol. 1983 Feb;14(2):108–126. doi: 10.1016/s0046-8177(83)80239-1. [DOI] [PubMed] [Google Scholar]
- Lauderdale B., Rohrbach M. S. The stimulation of angiotensin-converting enzyme induction in cultured monocytes by T4+ and T8+ lymphocytes. Ann N Y Acad Sci. 1986;465:46–55. doi: 10.1111/j.1749-6632.1986.tb18480.x. [DOI] [PubMed] [Google Scholar]
- Lieberman J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med. 1975 Sep;59(3):365–372. doi: 10.1016/0002-9343(75)90395-2. [DOI] [PubMed] [Google Scholar]
- Markowicz S., Engleman E. G. Granulocyte-macrophage colony-stimulating factor promotes differentiation and survival of human peripheral blood dendritic cells in vitro. J Clin Invest. 1990 Mar;85(3):955–961. doi: 10.1172/JCI114525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. N., Scadding J. G. Sarcoidosis. Am Rev Respir Dis. 1974 Dec;110(6):774–802. doi: 10.1164/arrd.1974.110.6P1.774. [DOI] [PubMed] [Google Scholar]
- NIH conference. Pulmonary sarcoidosis: a disease characterized and perpetuated by activated lung T-lymphocytes. Ann Intern Med. 1981 Jan;94(1):73–94. doi: 10.7326/0003-4819-94-1-73. [DOI] [PubMed] [Google Scholar]
- Norris D. A., Morris R. M., Sanderson R. J., Kohler P. F. Isolation of functional subsets of human peripheral blood monocytes. J Immunol. 1979 Jul;123(1):166–172. [PubMed] [Google Scholar]
- Okabe T., Suzuki A., Ishikawa H., Yotsumoto H., Ohsawa N. Cells originating from sarcoid granulomas in vitro. Am Rev Respir Dis. 1981 Nov;124(5):608–612. doi: 10.1164/arrd.1981.124.5.608. [DOI] [PubMed] [Google Scholar]
- Pinkston P., Bitterman P. B., Crystal R. G. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983 Apr 7;308(14):793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- Raff H. V., Picker L. J., Stobo J. D. Macrophage heterogeneity in man. A subpopulation of HLA-DR-bearing macrophages required for antigen-induced T cell activation also contains stimulators for autologous-reactive T cells. J Exp Med. 1980 Sep 1;152(3):581–593. doi: 10.1084/jem.152.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. W., McLemore T. L., Crystal R. G. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985 May;75(5):1488–1495. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach M. S., Conrad A. K. Comparison of the T lymphocyte-dependent induction of angiotensin-converting enzyme and leucine aminopeptidase in cultured human monocytes. Clin Exp Immunol. 1991 Mar;83(3):510–515. doi: 10.1111/j.1365-2249.1991.tb05670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach M. S., Deremee R. A. Serum angiotensin converting enzyme activity in sarcoidosis as measured by a simple radiochemical assay. Am Rev Respir Dis. 1979 May;119(5):761–767. doi: 10.1164/arrd.1979.119.5.761. [DOI] [PubMed] [Google Scholar]
- Rohrbach M. S. Metabolism and subcellular localization of angiotensin converting enzyme in cultured human monocytes. Biochem Biophys Res Commun. 1984 Nov 14;124(3):843–849. doi: 10.1016/0006-291x(84)91034-9. [DOI] [PubMed] [Google Scholar]
- Rohrbach M. S., Vuk-Pavlović Z., Martin W. J., DeLaval P., Genetet N. Induction of angiotensin converting enzyme in cultured human monocytes by a factor present in the bronchoalveolar lavage fluid of sarcoidosis patients. Sarcoidosis. 1988 Mar;5(1):17–23. [PubMed] [Google Scholar]
- Rohrbach M. S. [Glycine-1-14C]hippuryl-L-histidyl-L-leucine: a substrate for the radiochemical assay of angiotensin converting enzyme. Anal Biochem. 1978 Jan;84(1):272–276. doi: 10.1016/0003-2697(78)90510-9. [DOI] [PubMed] [Google Scholar]
- Silverstein E., Pertschuk L. P., Friedland J. Immunofluorescent localization of angiotensin converting enzyme in epithelioid and giant cells of sarcoidosis granulomas. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6646–6648. doi: 10.1073/pnas.76.12.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy P. B., Rohrbach M. S., Mann K. G. Functional prothrombinase complex assembly on isolated monocytes and lymphocytes. J Biol Chem. 1983 Jun 25;258(12):7264–7267. [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuk-Pavlović Z., Rohrbach M. S. An in vitro model for the induction of angiotensin-converting enzyme in sarcoidosis: possible parallels to the immune response. Clin Exp Immunol. 1988 Jun;72(3):499–504. [PMC free article] [PubMed] [Google Scholar]
- Yasaka T., Mantich N. M., Boxer L. A., Baehner R. L. Functions of human monocyte and lymphocyte subsets obtained by countercurrent centrifugal elutriation: differing functional capacities of human monocyte subsets. J Immunol. 1981 Oct;127(4):1515–1518. [PubMed] [Google Scholar]


