Abstract
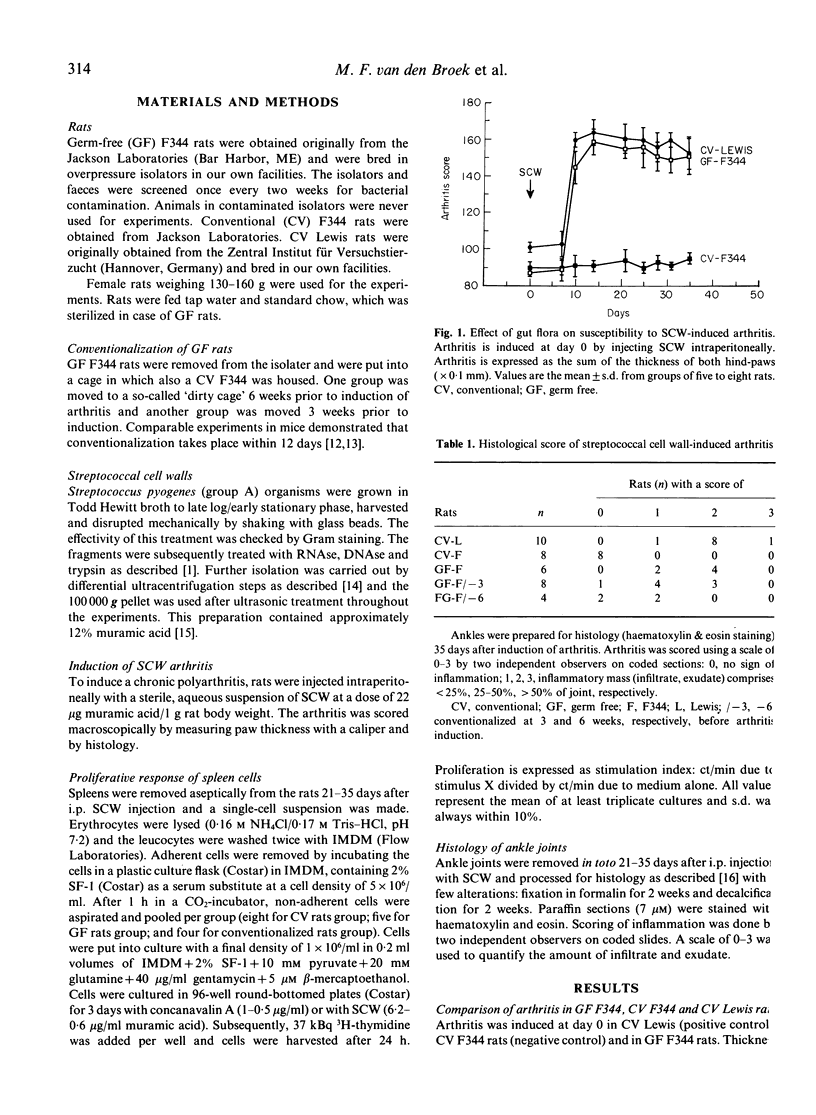
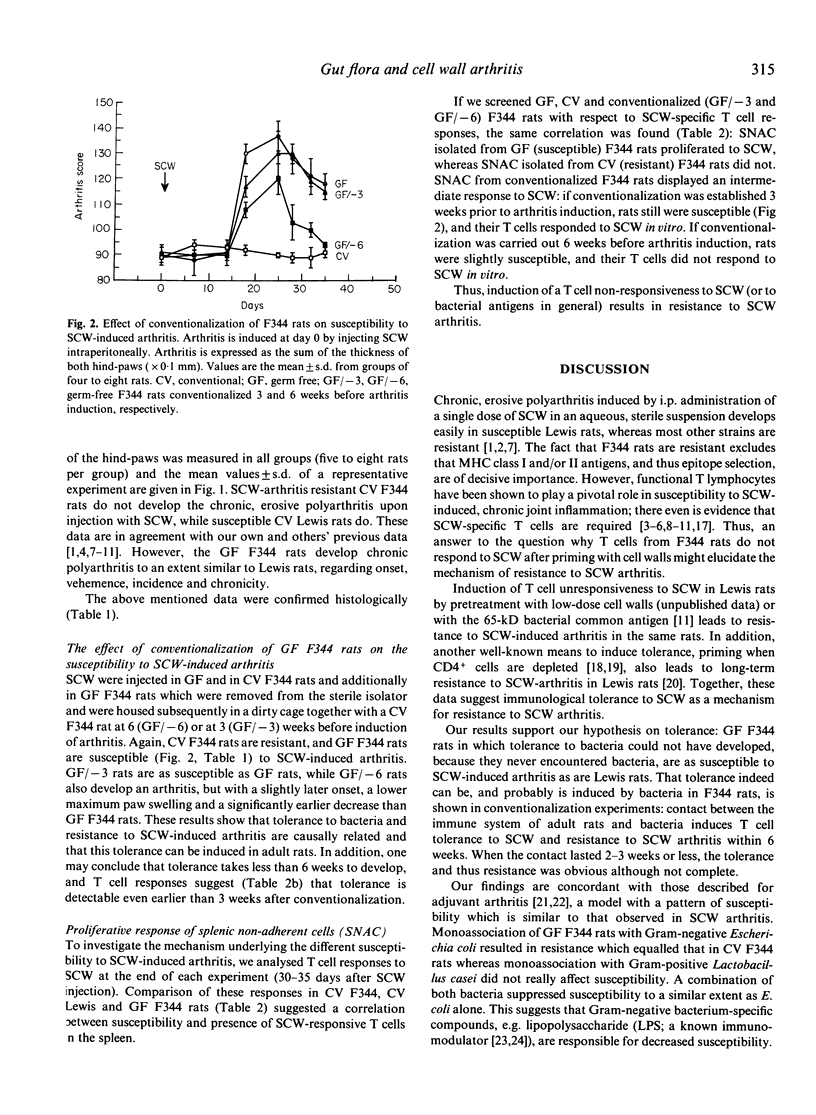
Streptococcal cell wall (SCW)-induced arthritis is a chronic, erosive polyarthritis that can be induced in susceptible Lewis rats by one i.p. injection of an aqueous, sterile suspension of SCW. F344 rats are resistant to chronic joint inflammation. Our previous studies showed a correlation between susceptibility to SCW-induced arthritis and the ability to mount SCW-specific T cell responses, suggesting tolerance to SCW as a putative mechanism. Here we prevented the induction of tolerance to bacterial epitopes in F344 rats by using them germ-free and analysed susceptibility to arthritis subsequently. In addition, we conventionalized germ-free F344 rats at different times before induction of arthritis. Our results show that germ-free F344 rats are susceptible to SCW-induced arthritis with a similar severity, chronicity, incidence and onset as Lewis rats. Moreover, T cells isolated from germ-free F344 rats were able to respond to SCW. Conventionalization dramatically moderates arthritis and makes T cells unresponsive to SCW again. Thus, in normal rats (F344) a state of tolerance to arthritogenic epitopes is induced (neonatally) and maintained through life by the bacterial flora, resulting in resistance to bacterium-induced arthritides. In arthritis-prone (Lewis) rats, this tolerance is deficient and/or easily broken.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B., Malone D. G., Wahl S. M., Calandra G. B., Wilder R. L. Role of the thymus in streptococcal cell wall-induced arthritis and hepatic granuloma formation. Comparative studies of pathology and cell wall distribution in athymic and euthymic rats. J Clin Invest. 1985 Sep;76(3):1042–1056. doi: 10.1172/JCI112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin R. J., Qin S. X., Wise M. P., Cobbold S. P., Waldmann H. Mechanisms of monoclonal antibody-facilitated tolerance induction: a possible role for the CD4 (L3T4) and CD11a (LFA-1) molecules in self-non-self discrimination. Eur J Immunol. 1988 Jul;18(7):1079–1088. doi: 10.1002/eji.1830180717. [DOI] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJoy S. Q., Ferguson K. M., Sapp T. M., Zabriskie J. B., Oronsky A. L., Kerwar S. S. Streptococcal cell wall arthritis. Passive transfer of disease with a T cell line and crossreactivity of streptococcal cell wall antigens with Mycobacterium tuberculosis. J Exp Med. 1989 Aug 1;170(2):369–382. doi: 10.1084/jem.170.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadzija O. A simple method for the quantitative determination of muramic acid. Anal Biochem. 1974 Aug;60(2):512–517. doi: 10.1016/0003-2697(74)90261-9. [DOI] [PubMed] [Google Scholar]
- Kohashi O., Kohashi Y., Takahashi T., Ozawa A., Shigematsu N. Suppressive effect of Escherichia coli on adjuvant-induced arthritis in germ-free rats. Arthritis Rheum. 1986 Apr;29(4):547–553. doi: 10.1002/art.1780290413. [DOI] [PubMed] [Google Scholar]
- Koopman J. P., Prins R. A., Mullink J. W., Welling G. W., Kennis H. M., Hectors M. P. Association of germfree mice with bacteria isolated from the intestinal tract of "normal" mice. Z Versuchstierkd. 1983;25(2):57–62. [PubMed] [Google Scholar]
- Koopman J. P., van Oeveren J. P., Janssen F. G. Use of combusted natural gas to cultivate the anaerobic bacterial flora from the cecum contents of mice. Appl Microbiol. 1973 Oct;26(4):584–588. doi: 10.1128/am.26.4.584-588.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Harada K., Shiba T. Correlation between the immunoadjuvant activities and pyrogenicities of synthetic N-acetylmuramyl-peptides or -amino acids. Biken J. 1976 Mar;19(1):9–13. [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M., WOOD F. D., McDANIEL E. G., DAFT F. S. Adjuvant arthritis induced in germ-free rats. Proc Soc Exp Biol Med. 1963 Jan;112:91–93. doi: 10.3181/00379727-112-27959. [DOI] [PubMed] [Google Scholar]
- Van den Broek M. F., Van de Langerijt L. G., Van Bruggen M. C., Billingham M. E., Van den Berg W. B. Treatment of rats with monoclonal anti-CD4 induces long-term resistance to streptococcal cell wall-induced arthritis. Eur J Immunol. 1992 Jan;22(1):57–61. doi: 10.1002/eji.1830220110. [DOI] [PubMed] [Google Scholar]
- WOOD F. D., PEARSON C. M. Protection of rats against adjuvant arthritis by bacterial lipoplysaccharides. Science. 1962 Aug 17;137(3529):544–545. doi: 10.1126/science.137.3529.544. [DOI] [PubMed] [Google Scholar]
- Wilder R. L., Allen J. B., Hansen C. Thymus-dependent and -independent regulation of Ia antigen expression in situ by cells in the synovium of rats with streptococcal cell wall-induced arthritis. Differences in site and intensity of expression in euthymic, athymic, and cyclosporin A-treated LEW and F344 rats. J Clin Invest. 1987 Apr;79(4):1160–1171. doi: 10.1172/JCI112933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder R. L., Allen J. B., Wahl L. M., Calandra G. B., Wahl S. M. The pathogenesis of group A streptococcal cell wall-induced polyarthritis in the rat. Comparative studies in arthritis resistant and susceptible inbred rat strains. Arthritis Rheum. 1983 Dec;26(12):1442–1451. doi: 10.1002/art.1780261205. [DOI] [PubMed] [Google Scholar]
- Yocum D. E., Allen J. B., Wahl S. M., Calandra G. B., Wilder R. L. Inhibition by cyclosporin A of streptococcal cell wall-induced arthritis and hepatic granulomas in rats. Arthritis Rheum. 1986 Feb;29(2):262–273. doi: 10.1002/art.1780290215. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B., van Beusekom H. J., van de Putte L. B., Zwarts W. A., van der Sluis M. Antigen handling in antigen-induced arthritis in mice: an autoradiographic and immunofluorescence study using whole joint sections. Am J Pathol. 1982 Jul;108(1):9–16. [PMC free article] [PubMed] [Google Scholar]
- van den Broek M. F., Hogervorst E. J., Van Bruggen M. C., Van Eden W., van der Zee R., van den Berg W. B. Protection against streptococcal cell wall-induced arthritis by pretreatment with the 65-kD mycobacterial heat shock protein. J Exp Med. 1989 Aug 1;170(2):449–466. doi: 10.1084/jem.170.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek M. F. Streptococcal cell wall-induced polyarthritis in the rat. Mechanisms for chronicity and regulation of susceptibility. APMIS. 1989 Oct;97(10):861–878. doi: 10.1111/j.1699-0463.1989.tb00491.x. [DOI] [PubMed] [Google Scholar]
- van den Broek M. F., van Bruggen M. C., Stimpson S. A., Severijnen A. J., van de Putte L. B., van den Berg W. B. Flare-up reaction of streptococcal cell wall induced arthritis in Lewis and F344 rats: the role of T lymphocytes. Clin Exp Immunol. 1990 Feb;79(2):297–306. doi: 10.1111/j.1365-2249.1990.tb05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek M. F., van Bruggen M. C., van de Putte L. B., van den Berg W. B. T cell responses to streptococcal antigens in rats: relation to susceptibility to streptococcal cell wall-induced arthritis. Cell Immunol. 1988 Oct 1;116(1):216–229. doi: 10.1016/0008-8749(88)90222-5. [DOI] [PubMed] [Google Scholar]


