Abstract
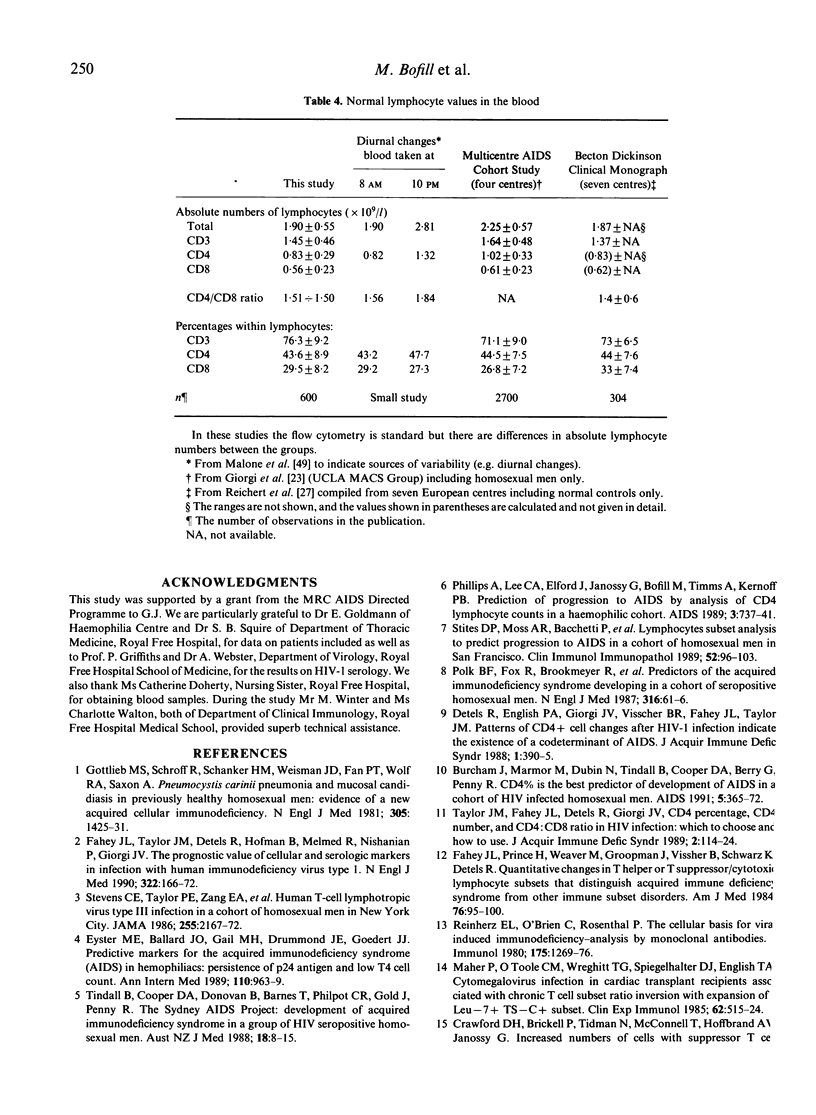
With the advent of standard flow cytometric methods using two-colour fluorescence on samples of whole blood, it is possible to establish the ranges of CD3, CD4 and CD8 T lymphocyte subsets in the routine laboratory, and also to assist the definition of HIV-1-related deviations from these normal values. In 676 HIV-1-seronegative individuals the lymphocyte subset percentages and absolute counts were determined. The samples taken mostly in the morning. The groups included heterosexual controls, people with various clotting disorders but without lymphocyte abnormalities as well as seronegative homosexual men as the appropriate controls for the HIV-1-infected groups. The stability of CD4% and CD8% values was demonstrated throughout life, and in children CD4 values less than 25% could be regarded as abnormal. The absolute counts of all T cell subsets decreased from birth until the age of 10 years. In adolescents and adults the absolute numbers (mean +/- s.d.) of lymphocytes, CD3, CD4 and CD8 cells were 1.90 +/- 0.55, 1.45 +/- 0.46, 0.83 +/- 0.29 and 0.56 +/- 0.23 x 10(9)/l, respectively. In patients with haemophilia A and B the mean values did not differ significantly. In homosexual men higher CD8 levels were seen compared with heterosexual men and 27% had an inverted CD4/CD8 ratio but mostly without CD4 lymphopenia (CD4 less than 0.4 x 10(9)/l). However, some healthy uninfected people were 'physiologically' lymphopenic without having inverted CD4/CD8 ratios. When the variations 'within persons' were studied longitudinally over a 5-year period, the absolute CD4 counts tended to be fixed at different levels. As a marked contrast, over 60% of asymptomatic HIV-1+ patients exhibited low CD4 counts less than 0.4 x 10(9)/l together with inverted CD4/CD8 ratios. Such combined changes among the heterosexual and HIV-1-seronegative homosexual groups were as rare as 1.4% and 3%, respectively. For this reason, when the lymphocyte tests show less than 0.4 x 10(9)/l CD4 count and a CD4/CD8 ratio of less than unity, the individuals need to be investigated further for chronicity of this disorder, the signs of viral infections such as HIV-1 and other causes of immunodeficiency.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. S., Potts R. C., Kardjito T., Grange J. M. T4 lymphopenia in patients with active pulmonary tuberculosis. Clin Exp Immunol. 1985 Apr;60(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- Beddall A. C., Al-Rubei K., Williams M. D., Hill F. G. Lymphocyte subset ratios and factor VIII usage in haemophilia. Arch Dis Child. 1985 Jun;60(6):530–536. doi: 10.1136/adc.60.6.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche S., Rouzioux C., Moscato M. L., Veber F., Mayaux M. J., Jacomet C., Tricoire J., Deville A., Vial M., Firtion G. A prospective study of infants born to women seropositive for human immunodeficiency virus type 1. HIV Infection in Newborns French Collaborative Study Group. N Engl J Med. 1989 Jun 22;320(25):1643–1648. doi: 10.1056/NEJM198906223202502. [DOI] [PubMed] [Google Scholar]
- Brody J. I., Pickering N. J., Fink G. B., Behr E. D. Altered lymphocyte subsets during cardiopulmonary bypass. Am J Clin Pathol. 1987 May;87(5):626–628. doi: 10.1093/ajcp/87.5.626. [DOI] [PubMed] [Google Scholar]
- Burcham J., Marmor M., Dubin N., Tindall B., Cooper D. A., Berry G., Penny R. CD4% is the best predictor of development of AIDS in a cohort of HIV-infected homosexual men. AIDS. 1991 Apr;5(4):365–372. doi: 10.1097/00002030-199104000-00002. [DOI] [PubMed] [Google Scholar]
- Campana D., Thompson J. S., Amlot P., Brown S., Janossy G. The cytoplasmic expression of CD3 antigens in normal and malignant cells of the T lymphoid lineage. J Immunol. 1987 Jan 15;138(2):648–655. [PubMed] [Google Scholar]
- Crawford D. H., Brickell P., Tidman N., McConnell I., Hoffbrand A. V., Janossy G. Increased numbers of cells with suppressor T cell phenotype in the peripheral blood of patients with infectious mononucleosis. Clin Exp Immunol. 1981 Feb;43(2):291–297. [PMC free article] [PubMed] [Google Scholar]
- Crowe S., Mills J., McGrath M. S. Quantitative immunocytofluorographic analysis of CD4 surface antigen expression and HIV infection of human peripheral blood monocyte/macrophages. AIDS Res Hum Retroviruses. 1987 Summer;3(2):135–145. doi: 10.1089/aid.1987.3.135. [DOI] [PubMed] [Google Scholar]
- Detels R., English P. A., Giorgi J. V., Visscher B. R., Fahey J. L., Taylor J. M., Dudley J. P., Nishanian P., Muñoz A., Phair J. P. Patterns of CD4+ cell changes after HIV-1 infection indicate the existence of a codeterminant of AIDS. J Acquir Immune Defic Syndr. 1988;1(4):390–395. [PubMed] [Google Scholar]
- Eyster M. E., Ballard J. O., Gail M. H., Drummond J. E., Goedert J. J. Predictive markers for the acquired immunodeficiency syndrome (AIDS) in hemophiliacs: persistence of p24 antigen and low T4 cell count. Ann Intern Med. 1989 Jun 15;110(12):963–969. doi: 10.7326/0003-4819-110-12-963. [DOI] [PubMed] [Google Scholar]
- Fahey J. L., Prince H., Weaver M., Groopman J., Visscher B., Schwartz K., Detels R. Quantitative changes in T helper or T suppressor/cytotoxic lymphocyte subsets that distinguish acquired immune deficiency syndrome from other immune subset disorders. Am J Med. 1984 Jan;76(1):95–100. doi: 10.1016/0002-9343(84)90756-3. [DOI] [PubMed] [Google Scholar]
- Fahey J. L., Taylor J. M., Detels R., Hofmann B., Melmed R., Nishanian P., Giorgi J. V. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990 Jan 18;322(3):166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- Falcão R. P., Ismael S. J., Donadi E. A. Age-associated changes of T lymphocyte subsets. Diagn Clin Immunol. 1987;5(4):205–208. [PubMed] [Google Scholar]
- Giorgi J. V., Cheng H. L., Margolick J. B., Bauer K. D., Ferbas J., Waxdal M., Schmid I., Hultin L. E., Jackson A. L., Park L. Quality control in the flow cytometric measurement of T-lymphocyte subsets: the multicenter AIDS cohort study experience. The Multicenter AIDS Cohort Study Group. Clin Immunol Immunopathol. 1990 May;55(2):173–186. doi: 10.1016/0090-1229(90)90096-9. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. S., Schroff R., Schanker H. M., Weisman J. D., Fan P. T., Wolf R. A., Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981 Dec 10;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Grady R. W., Akbar A. N., Giardina P. J., Hilgartner M. W., de Sousa M. Disproportionate lymphoid cell subsets in thalassaemia major: the relative contributions of transfusion and splenectomy. Br J Haematol. 1985 Apr;59(4):713–724. doi: 10.1111/j.1365-2141.1985.tb07367.x. [DOI] [PubMed] [Google Scholar]
- Hirschel B., Lazzarin A., Chopard P., Opravil M., Furrer H. J., Rüttimann S., Vernazza P., Chave J. P., Ancarani F., Gabriel V. A controlled study of inhaled pentamidine for primary prevention of Pneumocystis carinii pneumonia. N Engl J Med. 1991 Apr 18;324(16):1079–1083. doi: 10.1056/NEJM199104183241602. [DOI] [PubMed] [Google Scholar]
- Hoffman R. A., Kung P. C., Hansen W. P., Goldstein G. Simple and rapid measurement of human T lymphocytes and their subclasses in peripheral blood. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4914–4917. doi: 10.1073/pnas.77.8.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Coustan-Smith E., Campana D. The reliability of cytoplasmic CD3 and CD22 antigen expression in the immunodiagnosis of acute leukemia: a study of 500 cases. Leukemia. 1989 Mar;3(3):170–181. [PubMed] [Google Scholar]
- Janossy G., Pinching A. J., Bofill M., Weber J., McLaughlin J. E., Ornstein M., Ivory K., Harris J. R., Favrot M., Macdonald-Burns D. C. An immunohistological approach to persistent lymphadenopathy and its relevance to AIDS. Clin Exp Immunol. 1985 Feb;59(2):257–266. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Prentice H. G., Grob J. P., Ivory K., Tidman N., Grundy J., Favrot M., Brenner M. K., Campana D., Blacklock H. A. T lymphocyte regeneration after transplantation of T cell depleted allogeneic bone marrow. Clin Exp Immunol. 1986 Mar;63(3):577–586. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Tidman N., Papageorgiou E. S., Kung P. C., Goldstein G. Distribution of t lymphocyte subsets in the human bone marrow and thymus: an analysis with monoclonal antibodies. J Immunol. 1981 Apr;126(4):1608–1613. [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Lee C. A., Bofill M., Janossy G., Thomas H. C., Rizza C. R., Kernoff P. B. Relationships between blood product exposure and immunological abnormalities in English haemophiliacs. Br J Haematol. 1985 May;60(1):161–172. doi: 10.1111/j.1365-2141.1985.tb07397.x. [DOI] [PubMed] [Google Scholar]
- Lee C. A., Phillips A., Elford J., Miller E. J., Bofill M., Griffiths P. D., Kernoff P. B. The natural history of human immunodeficiency virus infection in a haemophilic cohort. Br J Haematol. 1989 Oct;73(2):228–234. doi: 10.1111/j.1365-2141.1989.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Levi F. A., Canon C., Blum J. P., Mechkouri M., Reinberg A., Mathe G. Circadian and/or circahemidian rhythms in nine lymphocyte-related variables from peripheral blood of healthy subjects. J Immunol. 1985 Jan;134(1):217–222. [PubMed] [Google Scholar]
- Lewis D. E., Gilbert B. E., Knight V. Influenza virus infection induces functional alterations in peripheral blood lymphocytes. J Immunol. 1986 Dec 15;137(12):3777–3781. [PubMed] [Google Scholar]
- Maher P., O'Toole C. M., Wreghitt T. G., Spiegelhalter D. J., English T. A. Cytomegalovirus infection in cardiac transplant recipients associated with chronic T cell subset ratio inversion with expansion of a Leu-7+ TS-C+ subset. Clin Exp Immunol. 1985 Dec;62(3):515–524. [PMC free article] [PubMed] [Google Scholar]
- Malone J. L., Simms T. E., Gray G. C., Wagner K. F., Burge J. R., Burke D. S. Sources of variability in repeated T-helper lymphocyte counts from human immunodeficiency virus type 1-infected patients: total lymphocyte count fluctuations and diurnal cycle are important. J Acquir Immune Defic Syndr. 1990;3(2):144–151. [PubMed] [Google Scholar]
- Margolick J. B., Scott E. R., Odaka N., Saah A. J. Flow cytometric analysis of gamma delta T cells and natural killer cells in HIV-1 infection. Clin Immunol Immunopathol. 1991 Jan;58(1):126–138. doi: 10.1016/0090-1229(91)90154-3. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- Moody D. J., Casavant C. H., Fulwyler M. J., McHugh T. M., Stites D. P. Multiparameter flow cytometric analysis of mononuclear cells from HIV-infected individuals. Cytometry Suppl. 1988;3:44–47. doi: 10.1002/cyto.990090810. [DOI] [PubMed] [Google Scholar]
- Pahwa S., Sia C., Harper R., Pahwa R. T lymphocyte subpopulations in high-risk infants: influence of age and blood transfusions. Pediatrics. 1985 Dec;76(6):914–917. [PubMed] [Google Scholar]
- Paxton H., Kidd P., Landay A., Giorgi J., Flomenberg N., Walker E., Valentine F., Fahey J., Gelman R. Results of the flow cytometry ACTG quality control program: analysis and findings. Clin Immunol Immunopathol. 1989 Jul;52(1):68–84. doi: 10.1016/0090-1229(89)90194-3. [DOI] [PubMed] [Google Scholar]
- Phillips A. N., Lee C. A., Elford J., Janossy G., Timms A., Bofill M., Kernoff P. B. Serial CD4 lymphocyte counts and development of AIDS. Lancet. 1991 Feb 16;337(8738):389–392. doi: 10.1016/0140-6736(91)91166-r. [DOI] [PubMed] [Google Scholar]
- Phillips A., Lee C. A., Elford J., Janossy G., Bofill M., Timms A., Kernoff P. B. Prediction of progression to AIDS by analysis of CD4 lymphocyte counts in a haemophilic cohort. AIDS. 1989 Nov;3(11):737–741. doi: 10.1097/00002030-198911000-00008. [DOI] [PubMed] [Google Scholar]
- Polk B. F., Fox R., Brookmeyer R., Kanchanaraksa S., Kaslow R., Visscher B., Rinaldo C., Phair J. Predictors of the acquired immunodeficiency syndrome developing in a cohort of seropositive homosexual men. N Engl J Med. 1987 Jan 8;316(2):61–66. doi: 10.1056/NEJM198701083160201. [DOI] [PubMed] [Google Scholar]
- Reichert T., DeBruyère M., Deneys V., Tötterman T., Lydyard P., Yuksel F., Chapel H., Jewell D., Van Hove L., Linden J. Lymphocyte subset reference ranges in adult Caucasians. Clin Immunol Immunopathol. 1991 Aug;60(2):190–208. doi: 10.1016/0090-1229(91)90063-g. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
- Sarasombath S., Suvatte V., Homchampa P. Kinetics of lymphocyte subpopulations in dengue hemorrhagic fever/dengue shock syndrome. Southeast Asian J Trop Med Public Health. 1988 Dec;19(4):649–656. [PubMed] [Google Scholar]
- Sattentau Q. J., Arthos J., Deen K., Hanna N., Healey D., Beverley P. C., Sweet R., Truneh A. Structural analysis of the human immunodeficiency virus-binding domain of CD4. Epitope mapping with site-directed mutants and anti-idiotypes. J Exp Med. 1989 Oct 1;170(4):1319–1334. doi: 10.1084/jem.170.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligmann M., Aractingi S., Oksenhendler E., Rabian C., Ferchal F., Gonnot G. CD4+ lymphocytopenia without HIV in patient with cryptococcal disease. Lancet. 1991 Jan 5;337(8732):57–58. doi: 10.1016/0140-6736(91)93382-j. [DOI] [PubMed] [Google Scholar]
- Shannon B. T., Roach J., Cheek-Luten M., Orosz C., Ruymann F. B. Progressive change in lymphocyte distribution and degree of hypergammaglobulinemia with age in children with hemophilia. J Clin Immunol. 1986 Mar;6(2):121–129. doi: 10.1007/BF00918744. [DOI] [PubMed] [Google Scholar]
- Squire S. B., Elford J., Bor R., Tilsed G., Salt H., Bagdades E. K., Janossy G., Griffiths P. D., Johnson M. A. Open access clinic providing HIV-I antibody results on day of testing: the first twelve months. BMJ. 1991 Jun 8;302(6789):1383–1386. doi: 10.1136/bmj.302.6789.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. E., Taylor P. E., Zang E. A., Morrison J. M., Harley E. J., Rodriguez de Cordoba S., Bacino C., Ting R. C., Bodner A. J., Sarngadharan M. G. Human T-cell lymphotropic virus type III infection in a cohort of homosexual men in New York City. JAMA. 1986 Apr 25;255(16):2167–2172. [PubMed] [Google Scholar]
- Stites D. P., Casavant C. H., McHugh T. M., Moss A. R., Beal S. L., Ziegler J. L., Saunders A. M., Warner N. L. Flow cytometric analysis of lymphocyte phenotypes in AIDS using monoclonal antibodies and simultaneous dual immunofluorescence. Clin Immunol Immunopathol. 1986 Feb;38(2):161–177. doi: 10.1016/0090-1229(86)90135-2. [DOI] [PubMed] [Google Scholar]
- Stites D. P., Moss A. R., Bacchetti P., Osmond D., McHugh T. M., Wang Y. J., Hebert S., Colfer B. Lymphocyte subset analysis to predict progression to AIDS in a cohort of homosexual men in San Francisco. Clin Immunol Immunopathol. 1989 Jul;52(1):96–103. doi: 10.1016/0090-1229(89)90196-7. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Fahey J. L., Detels R., Giorgi J. V. CD4 percentage, CD4 number, and CD4:CD8 ratio in HIV infection: which to choose and how to use. J Acquir Immune Defic Syndr. 1989;2(2):114–124. [PubMed] [Google Scholar]
- Thomas H. C., Brown D., Routhier G., Janossy G., Kung P. C., Goldstein G., Sherlock S. Inducer and suppressor T-cells in hepatitis B virus-induced liver disease. Hepatology. 1982 Mar-Apr;2(2):202–204. doi: 10.1002/hep.1840020203. [DOI] [PubMed] [Google Scholar]
- Tidman N., Janossy G., Bodger M., Granger S., Kung P. C., Goldstein G. Delineation of human thymocyte differentiation pathways utilizing double-staining techniques with monoclonal antibodies. Clin Exp Immunol. 1981 Sep;45(3):457–467. [PMC free article] [PubMed] [Google Scholar]
- Tindall B., Barker S., Donovan B., Barnes T., Roberts J., Kronenberg C., Gold J., Penny R., Cooper D. Characterization of the acute clinical illness associated with human immunodeficiency virus infection. Arch Intern Med. 1988 Apr;148(4):945–949. [PubMed] [Google Scholar]
- Tindall B., Cooper D. A., Donovan B., Barnes T., Philpot C. R., Gold J., Penny R. The Sydney AIDS Project: development of acquired immunodeficiency syndrome in a group of HIV seropositive homosexual men. Aust N Z J Med. 1988 Feb;18(1):8–15. doi: 10.1111/j.1445-5994.1988.tb02232.x. [DOI] [PubMed] [Google Scholar]
- Volberding P. A., Lagakos S. W., Koch M. A., Pettinelli C., Myers M. W., Booth D. K., Balfour H. H., Jr, Reichman R. C., Bartlett J. A., Hirsch M. S. Zidovudine in asymptomatic human immunodeficiency virus infection. A controlled trial in persons with fewer than 500 CD4-positive cells per cubic millimeter. The AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases. N Engl J Med. 1990 Apr 5;322(14):941–949. doi: 10.1056/NEJM199004053221401. [DOI] [PubMed] [Google Scholar]
- Wilson M., Rosen F. S., Schlossman S. F., Reinherz E. L. Ontogeny of human T and B lymphocytes during stressed and normal gestation: phenotypic analysis of umbilical cord lymphocytes from term and preterm infants. Clin Immunol Immunopathol. 1985 Oct;37(1):1–12. doi: 10.1016/0090-1229(85)90129-1. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]
- Wright J. J., Wagner D. K., Blaese R. M., Hagengruber C., Waldmann T. A., Fleisher T. A. Characterization of common variable immunodeficiency: identification of a subset of patients with distinctive immunophenotypic and clinical features. Blood. 1990 Nov 15;76(10):2046–2051. [PubMed] [Google Scholar]


