Abstract
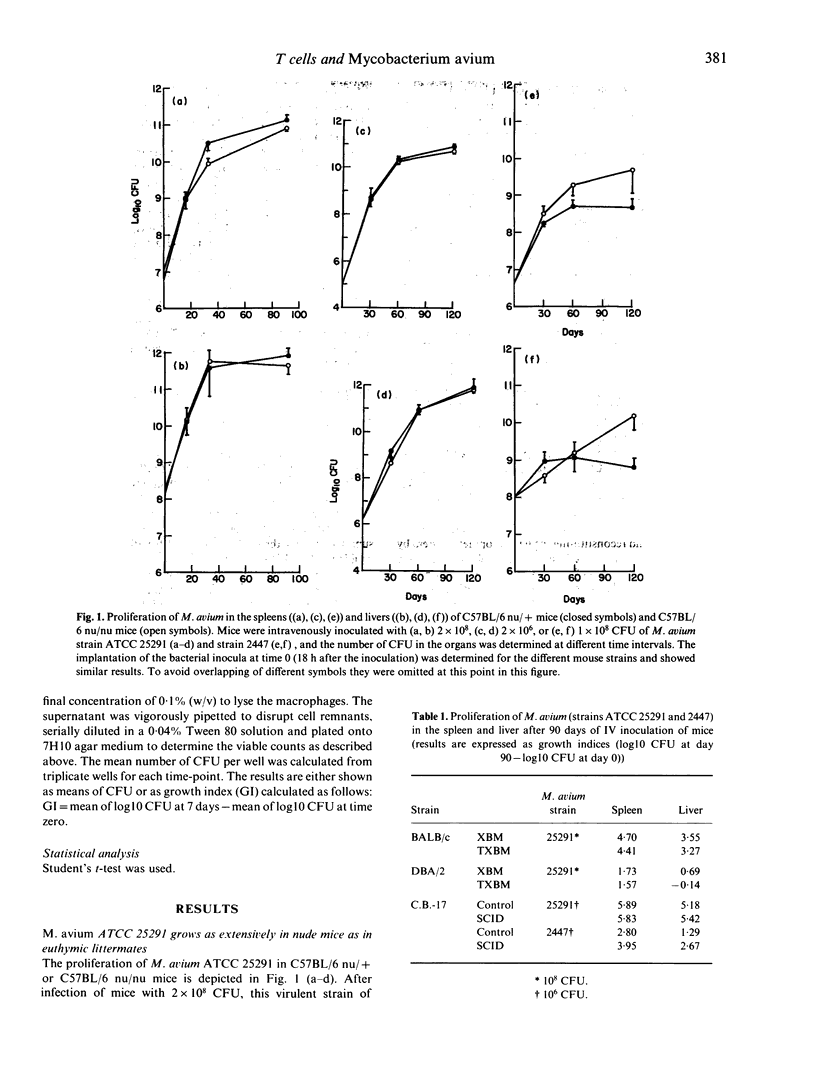
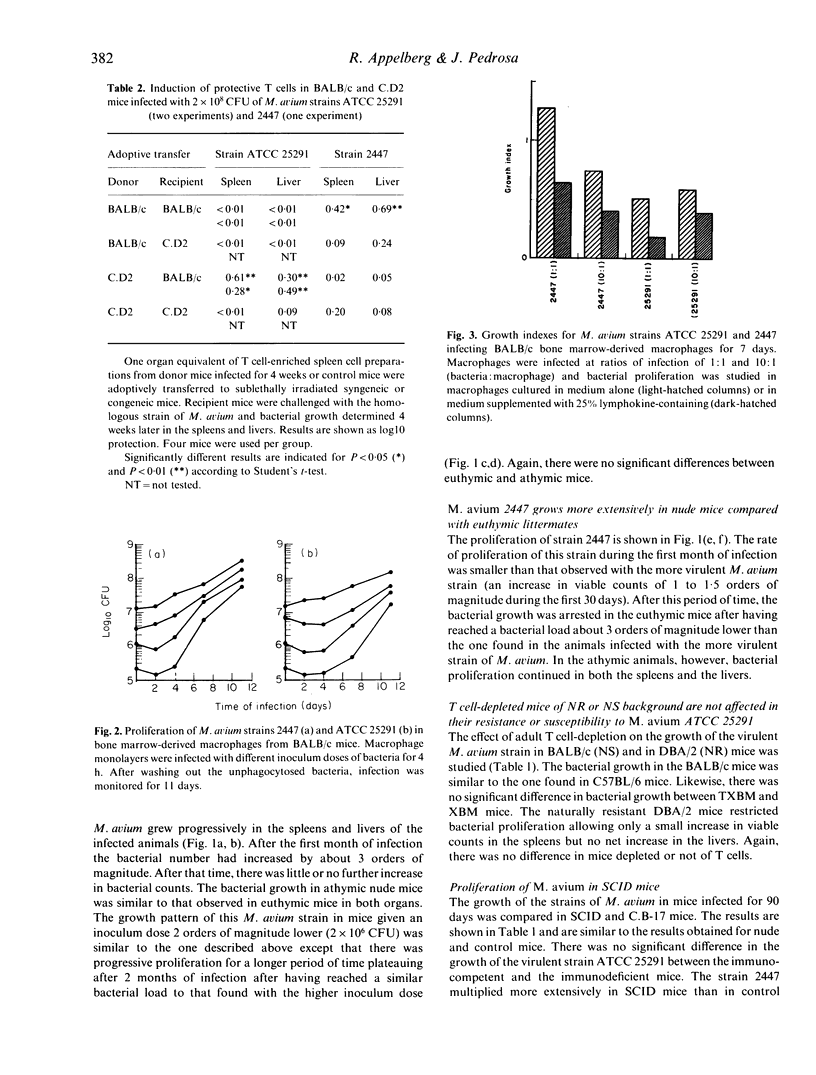
Mycobacterium avium is an opportunistic pathogen that infects individuals suffering from chronic lung disease or immunocompromised patients such as AIDS patients. Here we show that a highly virulent isolate of M. avium proliferated as extensively in T cell deficient as in immunocompetent mice. T cell deficient mice allowed a progressive growth of a less virulent AIDS-derived isolate of M. avium while immunocompetent mice arrested the growth of this isolate. Adoptive transfer of T cell enriched spleen cells between congenic strains of mice differing at the Bcg/Ity/Lsh locus showed that only naturally resistant BALB/c.Bcgr (C.D2) mice infected with the highly virulent strain of M. avium or the naturally susceptible BALB/c mice infected with the lower virulence isolate developed protective T cells and that these cells only mediated protection when transferred to naturally susceptible, but not to naturally resistant, mice. Both strains of M. avium proliferated in bone marrow-derived macrophages cultured in vitro and they were both susceptible to the bacteriostatic effects induced in the macrophages by crude lymphokines produced by concanavalin A-stimulated spleen cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Sarmento A. M. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990 Jun;80(3):324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Soares R., Ferreira P., Silva M. T. Induction of non-specific immunosuppression in mice by mycobacterial infections and its relationship to macrophage activation. Scand J Immunol. 1989 Aug;30(2):165–174. doi: 10.1111/j.1365-3083.1989.tb01198.x. [DOI] [PubMed] [Google Scholar]
- Bandeira A., Itohara S., Bonneville M., Burlen-Defranoux O., Mota-Santos T., Coutinho A., Tonegawa S. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor gamma delta. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman E., Apt A. S., Nickonenko B. V., Moroz A. M., Averbakh M. H., Skamene E. Genetic aspects of innate resistance and acquired immunity to mycobacteria in inbred mice. Springer Semin Immunopathol. 1988;10(4):319–336. doi: 10.1007/BF02053844. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Congdon C. C., Morrison N. E. Growth of mycobacterium bovis (BCG) in T lymphocyte-depleted mice. Infect Immun. 1975 Jan;11(1):57–64. doi: 10.1128/iai.11.1.57-64.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. II. Effect of adjuvant on the allergenicity and immunogenicity of heat-killed tubercle bacilli. Cell Immunol. 1970 Sep;1(3):266–275. doi: 10.1016/0008-8749(70)90048-1. [DOI] [PubMed] [Google Scholar]
- Crocker P. R., Blackwell J. M., Bradley D. J. Expression of the natural resistance gene Lsh in resident liver macrophages. Infect Immun. 1984 Mar;43(3):1033–1040. doi: 10.1128/iai.43.3.1033-1040.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Forget A., Pelletier M., Gervais F., Skamene E. Killing of Mycobacterium smegmatis by macrophages from genetically susceptible and resistant mice. J Leukoc Biol. 1990 Jan;47(1):25–30. doi: 10.1002/jlb.47.1.25. [DOI] [PubMed] [Google Scholar]
- Edwards D., Kirkpatrick C. H. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986 Nov;134(5):1062–1071. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- Gaugas J., Rees R. J. Enhancing effect of antilymphocytic serum on mycobacterial infections in mice. Nature. 1968 Jul 27;219(5152):408–409. doi: 10.1038/219408a0. [DOI] [PubMed] [Google Scholar]
- Good R. C. Opportunistic pathogens in the genus Mycobacterium. Annu Rev Microbiol. 1985;39:347–369. doi: 10.1146/annurev.mi.39.100185.002023. [DOI] [PubMed] [Google Scholar]
- Goto Y., Buschman E., Skamene E. Regulation of host resistance to Mycobacterium intracellulare in vivo and in vitro by the Bcg gene. Immunogenetics. 1989;30(3):218–221. doi: 10.1007/BF02421210. [DOI] [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Cellular mechanisms of genetically controlled host resistance to Mycobacterium bovis (BCG). J Immunol. 1983 Oct;131(4):1966–1972. [PubMed] [Google Scholar]
- Kaufmann S. H., Flesch I. E. The role of T cell--macrophage interactions in tuberculosis. Springer Semin Immunopathol. 1988;10(4):337–358. doi: 10.1007/BF02053845. [DOI] [PubMed] [Google Scholar]
- Lefford M. J. Mycobacterium lepraemurium infection of nude athymic (nu/nu) mice. Infect Immun. 1985 Jul;49(1):190–196. doi: 10.1128/iai.49.1.190-196.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveton C., Barnass S., Champion B., Lucas S., De Souza B., Nicol M., Banerjee D., Rook G. T-cell-mediated protection of mice against virulent Mycobacterium tuberculosis. Infect Immun. 1989 Feb;57(2):390–395. doi: 10.1128/iai.57.2.390-395.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner C. R., Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983 Dec;131(6):3006–3013. [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Olivier M., Tanner C. E. Susceptibilities of macrophage populations to infection in vitro by Leishmania donovani. Infect Immun. 1987 Feb;55(2):467–471. doi: 10.1128/iai.55.2.467-471.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988 May 15;140(10):3589–3593. [PubMed] [Google Scholar]
- Orme I. M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988 Dec;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Stokes R. W., Collins F. M. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect Immun. 1986 Oct;54(1):56–62. doi: 10.1128/iai.54.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. The dynamics of infection following BCG and Mycobacterium tuberculosis challenge in T-cell-deficient mice. Tubercle. 1987 Dec;68(4):277–283. doi: 10.1016/0041-3879(87)90068-7. [DOI] [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Champion B. R., Steele J., Varey A. M., Stanford J. L. I-A restricted activation by T cell lines of anti-tuberculosis activity in murine macrophages. Clin Exp Immunol. 1985 Feb;59(2):414–420. [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Properties of purified T cell subsets. I. In vitro responses to class I vs. class II H-2 alloantigens. J Exp Med. 1985 Dec 1;162(6):2068–2088. doi: 10.1084/jem.162.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stach J. L., Gros P., Forget A., Skamene E. Phenotypic expression of genetically-controlled natural resistance to Mycobacterium bovis (BCG). J Immunol. 1984 Feb;132(2):888–892. [PubMed] [Google Scholar]
- Stokes R. W., Collins F. M. Growth of Mycobacterium avium in activated macrophages harvested from inbred mice with differing innate susceptibilities to mycobacterial infection. Infect Immun. 1988 Sep;56(9):2250–2254. doi: 10.1128/iai.56.9.2250-2254.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes R. W., Collins F. M. Passive transfer of immunity of Mycobacterium avium in susceptible and resistant strains of mice. Clin Exp Immunol. 1990 Jul;81(1):109–115. doi: 10.1111/j.1365-2249.1990.tb05299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes R. W., Orme I. M., Collins F. M. Role of mononuclear phagocytes in expression of resistance and susceptibility to Mycobacterium avium infections in mice. Infect Immun. 1986 Dec;54(3):811–819. doi: 10.1128/iai.54.3.811-819.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima T., Collins F. M. T-cell-mediated immunity in persistent Mycobacterium intracellulare infections in mice. Infect Immun. 1988 Nov;56(11):2782–2787. doi: 10.1128/iai.56.11.2782-2787.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- Young L. S., Inderlied C. B., Berlin O. G., Gottlieb M. S. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986 Nov-Dec;8(6):1024–1033. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]
- Young L. S. Mycobacterium avium complex infection. J Infect Dis. 1988 May;157(5):863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]



