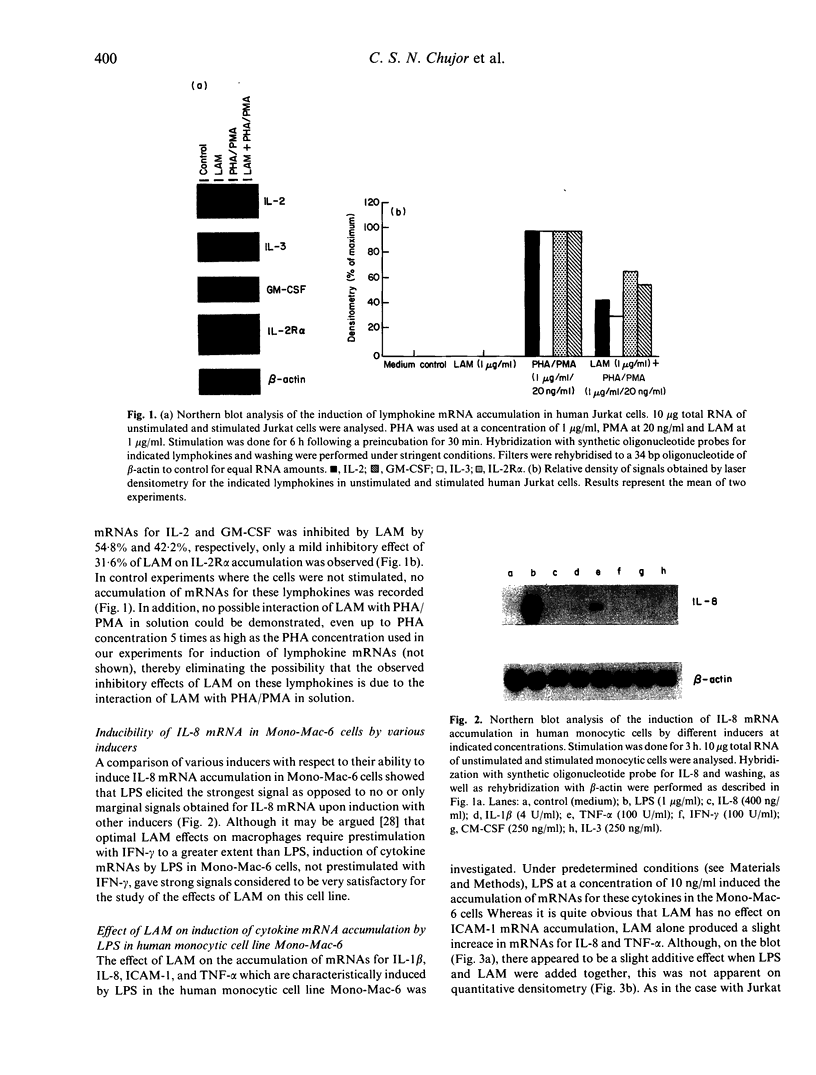
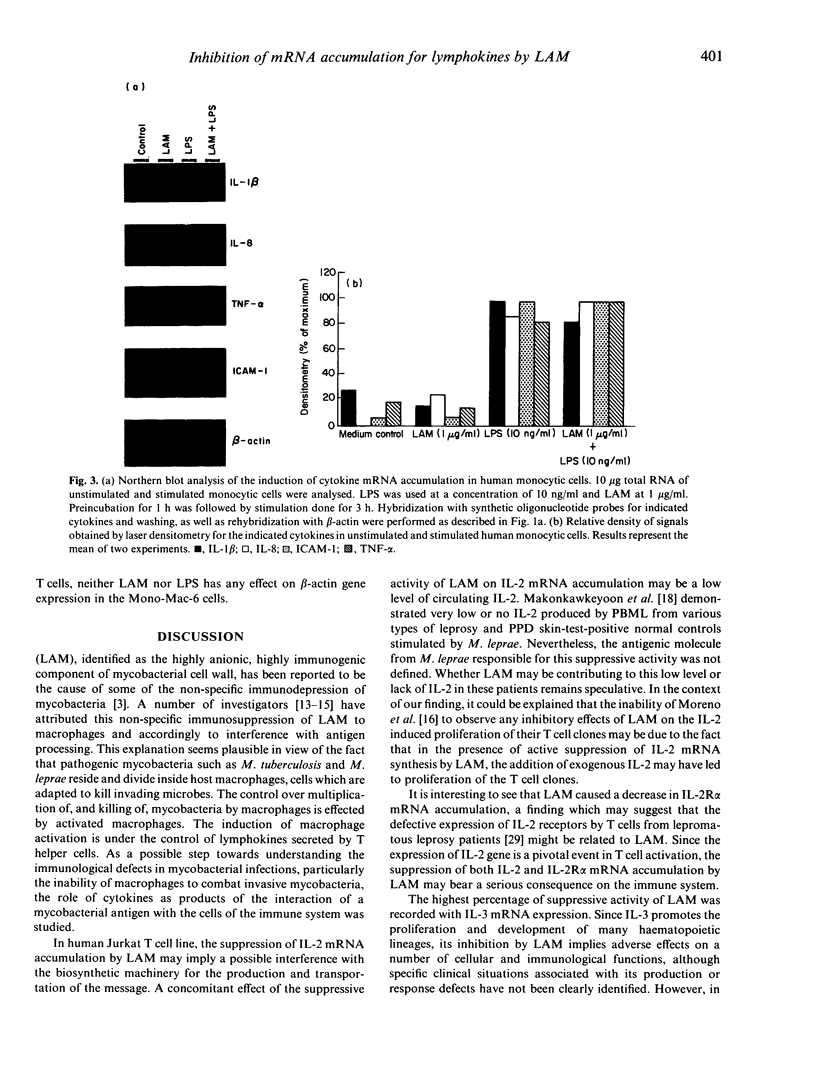
Abstract
The immunomodulatory effect of Mycobacterium tuberculosis-derived lipoarabinomannan (LAM) on mitogen/antigen-induced expression of mRNAs for a number of cytokines in human monocytic cell line Mono-Mac-6 and in T cell line Jurkat was investigated. Interestingly, LAM exhibited a down-regulatory effect on the accumulation of mRNAs for IL-2, IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-2 receptor alpha (IL-2R alpha) in T cells co-stimulated with phytohaemagglutinin-P (PHA) and 4 beta-phorbol-12-myristyl-13-acetate (PMA). In human Mono-Mac-6 cells. LAM has a weak inhibitory effect on the lipopolysaccharide (LPS)-induced mRNA accumulation for IL-1 beta, a slight stimulatory effect on mRNAs accumulation for IL-8 and tumour necrosis factor-alpha (TNF-alpha), but clearly no effect on mRNA accumulation for intercellular adhesion molecule-1 (ICAM-1). These findings imply that LAM may contribute to the immunologic defects associated with a number of mycobacterial infections by modulating these mediators.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr G. M., Rook G. A., Stanford J. L. Inhibition of the proliferative response of peripheral blood lymphocytes to mycobacterial or fungal antigens by co-stimulation with antigens from various mycobacterial species. Immunology. 1981 Nov;44(3):593–598. [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Fong S. J., Brennan P. J., Twomey P. E., Mazumder A., Modlin R. L. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990 Jul 1;145(1):149–154. [PubMed] [Google Scholar]
- Bjune G. In vitro lymphocyte stimulation in leprosy; simultaneous stimulation with Mycobacterium leprae antigens and phytohaemagglutinin. Clin Exp Immunol. 1979 Jun;36(3):479–487. [PMC free article] [PubMed] [Google Scholar]
- Chan J., Fan X. D., Hunter S. W., Brennan P. J., Bloom B. R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991 May;59(5):1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chujor C. S., Bernheimer H., Levis W. R., Schwerer B. Serum IgA1 and IgM antibodies against Mycobacterium leprae-derived phenolic glycolipid-I: a comparative study in leprosy patients and their contacts. Int J Lepr Other Mycobact Dis. 1991 Sep;59(3):441–449. [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J. Suppression of antigen and mitogen induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979 Dec;123(6):2689–2695. [PubMed] [Google Scholar]
- Gaylord H., Brennan P. J. Leprosy and the leprosy bacillus: recent developments in characterization of antigens and immunology of the disease. Annu Rev Microbiol. 1987;41:645–675. doi: 10.1146/annurev.mi.41.100187.003241. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Levis W. R., Meeker H. C., Schuller-Levis G., Sersen E., Brennan P. J., Fried P. Mycobacterial carbohydrate antigens for serological testing of patients with leprosy. J Infect Dis. 1987 Nov;156(5):763–769. doi: 10.1093/infdis/156.5.763. [DOI] [PubMed] [Google Scholar]
- Makonkawkeyoon S., Kasinrerk W. In vitro suppression of interleukin 2 production by Mycobacterium leprae antigen. Clin Exp Immunol. 1989 Jun;76(3):398–403. [PMC free article] [PubMed] [Google Scholar]
- Makonkawkeyoon S., Kasinrerk W., Supajatura V., Hirunpetcharat C., Vithayasai V. Immunologic defects in leprosy patients. II. Interleukin 1, interleukin 2, and interferon production in leprosy patients. Int J Lepr Other Mycobact Dis. 1990 Jun;58(2):311–318. [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Mehra V., Convit J., Rubinstein A., Bloom B. R. Activated suppressor T cells in leprosy. J Immunol. 1982 Nov;129(5):1946–1951. [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Rothman W., Reinherz E., Schlossman S. F., Bloom B. R. Delineation of a human T cell subset responsible for lepromin-induced suppression in leprosy patients. J Immunol. 1980 Sep;125(3):1183–1188. [PubMed] [Google Scholar]
- Mohagheghpour N., Gelber R. H., Larrick J. W., Sasaki D. T., Brennan P. J., Engleman E. G. Defective cell-mediated immunity in leprosy: failure of T cells from lepromatous leprosy patients to respond to Mycobacterium leprae is associated with defective expression of interleukin 2 receptors and is not reconstituted by interleukin 2. J Immunol. 1985 Aug;135(2):1443–1449. [PubMed] [Google Scholar]
- Molloy A., Gaudernack G., Levis W. R., Cohn Z. A., Kaplan G. Suppression of T-cell proliferation by Mycobacterium leprae and its products: the role of lipopolysaccharide. Proc Natl Acad Sci U S A. 1990 Feb;87(3):973–977. doi: 10.1073/pnas.87.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Mehlert A., Lamb J. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin Exp Immunol. 1988 Nov;74(2):206–210. [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Taverne J., Mehlert A., Bate C. A., Brealey R. J., Meager A., Rook G. A., Playfair J. H. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989 May;76(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- Nath I., Singh R. The suppressive effect of M. leprae on the in vitro proliferative responses of lymphocytes from patients with leprosy. Clin Exp Immunol. 1980 Sep;41(3):406–414. [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Kaplan G., Levy E., Sarno E. N., Kushner P., Granelli-Piperno A., Vieira L., Colomer Gould V., Levis W., Steinman R. Defective gamma interferon production in leprosy. Reversal with antigen and interleukin 2. J Exp Med. 1983 Dec 1;158(6):2165–2170. doi: 10.1084/jem.158.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Schwerer B., Chujor C. S., Bernheimer H., Radl J., Haaijman J. J., Meeker H. C., Sersen G., Levis W. R. IgA antibodies against phenolic glycolipid I from Mycobacterium leprae in serum of leprosy patients and contacts: subclass distribution and relation to disease activity. Clin Immunol Immunopathol. 1989 Nov;53(2 Pt 1):202–211. doi: 10.1016/0090-1229(89)90050-0. [DOI] [PubMed] [Google Scholar]
- Touw J., Stoner G. L., Belehu A. Effect of Mycobacterium leprae on lymphocyte proliferation: suppression of mitogen and antigen responses of human peripheral blood mononuclear cells. Clin Exp Immunol. 1980 Sep;41(3):397–405. [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Thiel E., Fütterer A., Herzog V., Wirtz A., Riethmüller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988 Mar 15;41(3):456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]