Abstract
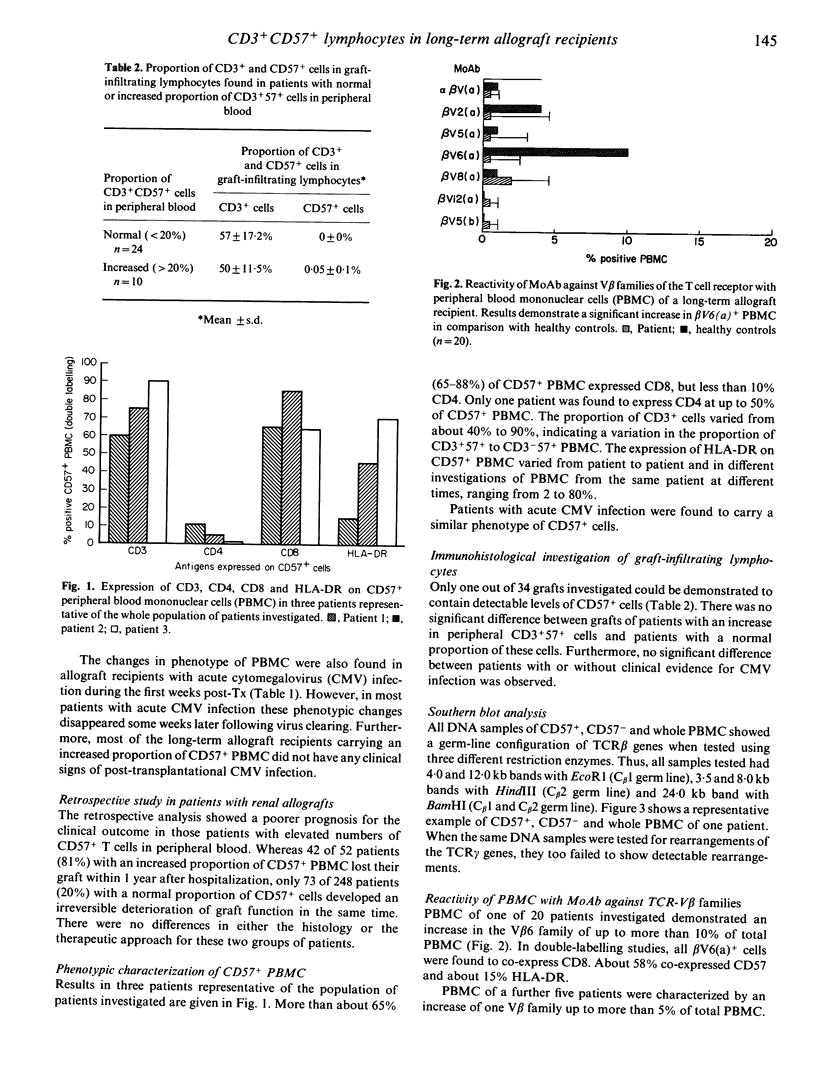
Cytofluorometric investigation of peripheral blood lymphocytes in 380 long-term (greater than 1 year posttransplantation) allograft recipients showed a significant increase in the proportion of CD3+57+ lymphocytes (greater than 20%) in 20% of patients with renal allografts, 66% of patients with cardiac allografts and 44% of patients with liver allografts. Most of these CD3+57+ cells expressed the CD8 antigen and a variable proportion the HLA-DR antigen. A retrospective analysis showed a poorer prognosis for the clinical outcome in those patients with elevated numbers of CD3+57+ cells in peripheral blood. However, CD57+ lymphocytes could rarely be detected in renal infiltrates by immunohistology. Using the Southern blot technique to analyse the T cell receptor rearrangement of separated CD57+ cells, no clonal or oligoclonal expansion of T cell clones could be detected. Nevertheless, there might be a bias towards the use of particular TCR-V beta gene families in at least some patients, as shown by analysis with monoclonal antibodies. In summary, CD57+ T cells are not likely to be directly involved in the rejection process. The data support the idea of a polyclonal and/or superantigen-driven expansion, but not of an antigen-driven expansion of these cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Cooper M. D., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. I. Two distinct phenotypes of human NK cells with different cytotoxic capability. J Immunol. 1982 Oct;129(4):1752–1757. [PubMed] [Google Scholar]
- Collins M. K., Kissonerghis A. M., Dunne M. J., Watson C. J., Rigby P. W., Owen M. J. Transcripts from an aberrantly re-arranged human T-cell receptor beta-chain gene. EMBO J. 1985 May;4(5):1211–1215. doi: 10.1002/j.1460-2075.1985.tb03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Fregona I., Guttmann R. D., Jean R. HNK-1+ (Leu-7) and other lymphocyte subsets in long-term survivors with renal allotransplants. Transplantation. 1985 Jan;39(1):25–29. [PubMed] [Google Scholar]
- Kappler J. W., Wade T., White J., Kushnir E., Blackman M., Bill J., Roehm N., Marrack P. A T cell receptor V beta segment that imparts reactivity to a class II major histocompatibility complex product. Cell. 1987 Apr 24;49(2):263–271. doi: 10.1016/0092-8674(87)90567-8. [DOI] [PubMed] [Google Scholar]
- Künemund V., Jungalwala F. B., Fischer G., Chou D. K., Keilhauer G., Schachner M. The L2/HNK-1 carbohydrate of neural cell adhesion molecules is involved in cell interactions. J Cell Biol. 1988 Jan;106(1):213–223. doi: 10.1083/jcb.106.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea T., Vartdal F., Davies C., Ugelstad J. Magnetic monosized polymer particles for fast and specific fractionation of human mononuclear cells. Scand J Immunol. 1985 Aug;22(2):207–216. doi: 10.1111/j.1365-3083.1985.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Lefranc M. P., Rabbitts T. H. Two tandemly organized human genes encoding the T-cell gamma constant-region sequences show multiple rearrangement in different T-cell types. Nature. 1985 Aug 1;316(6027):464–466. doi: 10.1038/316464a0. [DOI] [PubMed] [Google Scholar]
- Legendre C. M., Guttmann R. D., Hou S. K., Jean R. Two-color immunofluorescence and flow cytometry analysis of lymphocytes in long-term renal allotransplant recipients: identification of a major Leu-7+/Leu-3+ subpopulation. J Immunol. 1985 Aug;135(2):1061–1066. [PubMed] [Google Scholar]
- Leroy E., Calvo C. F., Divine M., Gourdin M. F., Baujean F., Ben Aribia M. H., Mishal Z., Vernant J. P., Farcet J. P., Senik A. Persistence of T8+/HNK-1+ suppressor lymphocytes in the blood of long-term surviving patients after allogeneic bone marrow transplantation. J Immunol. 1986 Oct 1;137(7):2180–2189. [PubMed] [Google Scholar]
- Leroy E., Madariaga L., Ben Aribia M., Mishal Z., Theodorou I., Rochant H., Vernant J. P., Senik A. Abnormally expanded CD8+/Leu-7+ lymphocytes persisting in long-term bone marrow-transplanted patients are resting pre-cytotoxic T-lymphocytes. Exp Hematol. 1990 Aug;18(7):770–774. [PubMed] [Google Scholar]
- Morel P. A., Livingstone A. M., Fathman C. G. Correlation of T cell receptor V beta gene family with MHC restriction. J Exp Med. 1987 Aug 1;166(2):583–588. doi: 10.1084/jem.166.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Germain R. N. Predictable acquisition of a new MHC recognition specificity following expression of a transfected T-cell receptor beta-chain gene. Nature. 1987 Sep 17;329(6136):256–259. doi: 10.1038/329256a0. [DOI] [PubMed] [Google Scholar]



