Abstract
Objective:
To examine common herbal supplements, explore potential risks associated with herbal use, and provide recommendations to the athletic trainer regarding patient care issues.
Data Sources:
We searched MEDLINE, SPORT Discus, CINAHL, and Academic Search Elite databases 1990–2000 using the key words herbals, regulation, supplements, toxicity, and adulteration.
Data Synthesis:
The use of herbal products continues to grow. While the origins of some medications and herbal supplements are similar, clinical testing and understanding of most herbal remedies is lacking. Some herbal products may prove useful in an athletic setting; however, current United States Food and Drug Administration (FDA) regulations do not ensure safe and effective products. A descriptive review focusing on specific considerations for the athletic trainer is provided.
Conclusions/Recommendations:
Despite their increasing tendency to seek natural therapies, athletes need to be aware that “natural” does not equal “safe.” Athletes are entitled to know that most herbs are not proven safe or effective under current FDA standards. The athletic trainer must be able to provide honest, unbiased information when educating athletes regarding herbal supplements.
Keywords: herbals, botanicals, toxicity, adulteration, regulation
Recent increases in the availability and popularity of herbal supplements and complementary health care products have created an environment of hyperbole and misinformation for patients and health care providers alike. Athletic trainers and other health care professionals must be able to distinguish fact from fiction and direct their patients to appropriate sources when trying to determine the efficacy and potential dangers of these products. Reports indicate that Americans spend in excess of $12 billion annually on vitamins, minerals, herbals, sports supplements, and specialty supplements.1
The prevalence of herbal use is largely unstudied.2–4 It is estimated that 33% of patients have used at least 1 unconventional treatment in the past year.3 Eisenberg et al3 defined unconventional treatments as medical interventions not taught widely at United States (US) medical schools or generally available at US hospitals; examples include acupuncture, chiropractic, and massage therapy. In another study, Eliason et al5 found that 52% of patients have taken 1 or more dietary supplements during the past year and that the media is their primary source of information about the supplements. Compounding this increase in availability and use are government regulations that limit the Food and Drug Administration's (FDA) ability to regulate any product labeled as a supplement. The 1994 Dietary Supplement and Health Education Act (DSHEA) allows companies to promote supplements with claims of improved “function and health” as long as they make no claims to affect disease.6
Athletes demonstrate a greater willingness to use supplement products when compared with their nonathlete counterparts.7 The athletic trainer is often called upon to serve as an educational resource for athletes wishing to learn more about herbal supplements. Herbal products are vigorously marketed to both competitive and recreational athletes with claims of performance gains and improved health and wellness. This review examines the regulation of herbal supplements, explores potential risks associated with herbal use, and provides recommendations to the athletic trainer regarding patient care issues.
REGULATION
Food and Drug Administration
The regulation of herbal products has proven to be a confusing blend of public safety issues, varied international guidelines, advertising hyperbole, and partisan politics. In the US, the regulation of drugs, food, and cosmetics is the job of the FDA, which helps assure the public that drugs are safe and effective and have been subject to scientific scrutiny. In 1962, the FDA required that all drugs be evaluated for safety and efficacy.8 To avoid the burden of proof associated with FDA approval, herbal manufacturers began to label herbs as “foods” and sell them in health food stores. The FDA maintains a list of products “Generally Recognized as Safe” (GRAS). Appoximately 250 herbs appear on this list, but these are herbs used for food flavoring and not for medicinal purposes. Currently, only a handful of herbs have been shown safe and effective based on a 1990 FDA review of over-the-counter drugs (Table 1).9
Table 1.
Common Herbs With FDA Approval2

It is estimated that more than 1400 herbs are commonly sold and promoted for medicinal uses worldwide.1,2,4 Historically, US manufacturers have had little incentive to seek FDA approval due to the costs associated with drug research. In turn, herbs reviewed by the FDA have only been examined within a very narrow definition of medicinal actions.9 This left the public largely unaware of which products were safe, effective, or both safe and effective.
In 1993, the FDA distributed an advance notice of proposed rule making that addressed the herbal and supplement industry. The report discussed instances of herb-related deaths and concerns about toxicities. The consensus in Washington was that stricter regulation was on the way.11 What resulted was an unanticipated public and political backlash from consumers who thought that their access to herbals and supplements would be taken away. At the urging of the supplement industry, Congress was deluged with millions of letters and faxes. The result was DSHEA,6 a political compromise that has limited the FDA's influence on herbal products.
This legislation allows herbal products to be sold without any testing for efficacy. Companies cannot make claims on an herb's ability to cure a disease, but they may make claims about how a supplement affects the “structure” and “function” of the body. This nebulous language has not helped to clear the confusion surrounding the herbal industry. For example, an herb could not be claimed to cure inflammation but could be claimed to promote healthy joints (structure and function). Manufacturers can make structure and function claims as long as they provide a disclaimer stating that their products have not been reviewed by the FDA and are not intended to be used as drugs.6 Under the current legislation, supplement makers do not have to prove a product is safe; the FDA has the burden of proving a product is unsafe. The FDA can only take action if a product is found to present a significant or unreasonable risk of illness or injury. Further confounding the herbal landscape are studies that show consumers tend to believe that products sold in a pill form have been reviewed for safety by the FDA, despite required label disclaimers.11,12
International Considerations
Given the limited number of herbs with FDA approval, considerable information on the use and dosage of herbals comes from European guidelines.4 These guidelines vary considerably from one country to the next and often rely on the historical use of a product. Substances are often accepted under the doctrine of reasonable certainty because they have a long history of use. This philosophy is similar to the World Health Organization's Guidelines for the Assessment of Herbal Medicines, which state that a substance's historical use is a valid way to document safety and efficacy in the absence of scientific evidence to the contrary.13 A long history of use may allow for safety information to be gathered; however, it may do little to assess efficacy.
The most often cited European guidelines are those of the German Commission E. Beginning in 1978, the German Commission E has reviewed clinical literature (including clinical trials and case studies) on more than 1400 herbal drugs.2,4 The commission has produced more than 300 monographs on common herbal remedies. However, these monographs must be used with caution given their reliance on historical bibliographic information that may or may not include data gathered from clinical trials.
Athletic trainers must also be aware of the availability of Chinese herbal preparations and Ayurvedic herbal products. Ayurvedic herbs are used in the Ayurveda medical system that is common in India. Currently, about 300 herbs are used in general practice in traditional Chinese medicine. Often these herbs are sold in preparations that contain multiple herbs. For example, Chinese black balls contain up to 20 different herbs and are used to treat everything from arthritis to asthma.14 Both Chinese and Ayurvedic products are largely unregulated, and some do not list ingredients in English. The concerns for athletes range from positive drug testing to the risk of toxicity due to unknown ingredients.
RISK FACTORS
Concentration and Purity
The risks associated with the use of herbal remedies and supplements can range from minor skin irritations to death. Determining the safety and efficacy of herbal products continues to be difficult because the FDA, herbal supplement manufacturers, and herbal experts disagree on how to interpret the varying evidence available for many types of herbal remedies.15 Owing to the limited regulation of herbs, patients are often unable to tell how much of the herb or which part of the herb is contained within a given product.1 Both the scientific literature and the media have reported concerns with herbal products. In 1995, Consumer Reports1 tested 10 brands of ginseng and found substantial variations in concentration among brands. In March 1998, the Good Housekeeping Institute tested 9 brands of St. John's wort and found a significant variation in the amount of active ingredient. The Los Angeles Times also tested St. John's wort in 1998 and found that 7 of the 10 brands tested were low in the amount of purported active ingredient.1 An herb's ability to create a physiologic response depends upon the availability of a specific chemical constituent. The variability of these active ingredients is of concern because the most profound risks of herbal product use are toxicity and adverse reactions, herb-drug interactions, and adulteration of herbal products.
Toxicities and Adverse Reactions
Numerous cases of toxicity have been linked to the use of herbal products.16–47 The resulting problems range from minor adverse reactions to serious physical disabilities and death. Adverse reactions have been reported in athletic training settings. Myers et al47 reported syncope and atypical chest pain in an intercollegiate wrestler after ingestion of an over-the-counter metabolic stimulant containing Chinese herbal extracts. This particular stimulant (Ripped Fuel, Twinlab Inc, Ronkonkoma, NY) contained ma huang (ephedrine) and caffeine. The stimulant effects were compounded by the athlete's aggressive weight-loss techniques. Winterstein (unpublished data, 2000) described a 19-year-old female soccer player with an episode of syncope and tachycardia after ingestion of an over-the-counter stimulant containing ma huang, guarana, and caffeine. This athlete had also been severely restricting calories to lose weight. These cases illustrate a common problem: athletes taking products that are marketed as “metabolism boosters” that contain “natural” herbal ingredients.
The herb ma huang and all ephedrine alkaloids have received considerable attention from the FDA. More than 15 deaths have been attributed to the use of ephedrine alkaloid products.47 In 1996, the FDA issued a warning to consumers to avoid nutritional supplements containing ephedrine.48 In 1997, the FDA proposed the use of warning labels addressing the adverse effects of ephedrine, banning products containing more than 8 mg per serving, and eliminating products containing combinations of ephedrine and caffeine.49 The FDA received 14 775 public comments in response to the 1997 ephedrine alkaloids proposal. The Center for Food Safety and Applied Nutrition and the FDA's Center for Drug Evaluation and Research have examined hundreds of reports from consumers who have experienced adverse effects from supplements containing ephedrine. Despite the volume of adverse reactions, the FDA has yet to impose the 1997 proposed rule changes and continues to meet resistance and political pressure from herbal manufacturers.50
In addition to recent concerns over products containing ma huang and ephedrine alkaloids, the FDA has recently made public additional concerns over botanical products containing aristolochic acid. Aristolochic acid is a known carcinogen and nephrotoxin; side effects include interstitial renal fibrosis and renal failure. A recent FDA report51 identified 76 botanicals known or suspected of containing aristolochic acid and 92 botanicals believed adulterated with aristolochic acid. Products containing a large amount of this substance may produce rapid-onset toxicity. However, the effects of long-term use are unknown. The first indication of adverse effects may be irreversible, such as renal failure.51
The toxicity and adverse effects of some common herbs that athletes may come in contact with or may already be using are outlined in Table 2. These are categorized as stimulants or energy boosters, weight-control agents, pain-control (ie, analgesics) and wound-healing agents, anti-inflammatories, antidepressants, and sleep aids.
Table 2.
Toxicity and Adverse Effects of Common Herbs
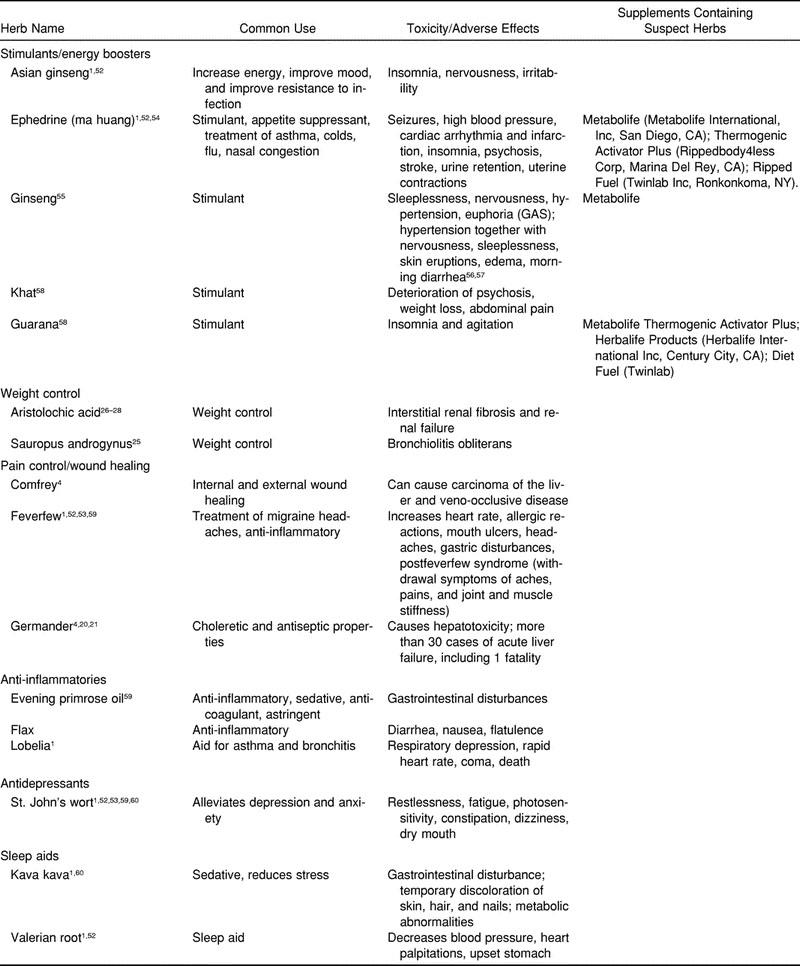
Some herbal drugs on the market have been found to be relatively safe and free of serious adverse effects when taken in specific dosages (Table 3).4 These herbs have undergone clinical trials, have been reviewed by German Commission E, or have a history of safe consumption.
Table 3.
Potentially Beneficial Herbs

Despite safety claims, patients and health care providers should be aware that abuse of dosages and problems with adulteration may render an otherwise safe herbal product dangerous. Ginseng, although considered by many sources to be relatively safe, had a high incidence of adverse effects in a 2-year study by Siegel.56 The long-term use of ginseng has been associated with central nervous system excitation and arousal.57 The long-term effects have been labeled ginseng abuse syndrome.56,57
Herb-Drug Interactions
Patients often neglect to mention herbs when asked by their health care providers about medications taken on a regular basis because they (1) assume that herbs are natural,3 (2) are embarrassed by the reason they are taking the herb, or (3) feel their physician will not approve of their herbal use.52,69 However, not informing health care providers about herbal use places patients at risk because of the possible interactions between drugs and herbs (Table 4). Owing to a lack of research showing which herb-drug combinations athletes are likely to consume, we have included examples from the literature of over-the-counter and prescription drugs that athletes may come in contact with or may already be using. The known effects of using prescription drugs and herbs in combination are that herbs can “mimic, magnify, or oppose the effect of the drugs.”69,70 Athletic trainers need to be sensitive, form a trusting relationship with athletes, and ask about the possible use of herbal products in a nonthreatening manner.
Table 4.
Drug Interactions With Common Herbs

Product Adulteration
Despite attempts to improve manufacturing processes, reports on product adulteration, contamination, or both are common in the literature.1,24,46,53,59,70,73–97 Adulteration cases often include Ayurvedic and Chinese herbal medicines with multiple ingredients; these products have been contaminated with lead, arsenic, and other highly toxic substances. The British National Poisons Information Service identified herbal preparations containing toxic levels of lead, zinc, mercury, arsenic, aluminum, and tin. The individuals who had ingested the herbals had blood concentrations of the heavy metals elevated by 2 to 10 times the upper limit of normal physiologic values.83
One report of herbal product adulteration showed more than 48 cases of renal poisoning when the patients thought they were taking fang ji. In actuality, patients were taking guang fang ji. The problem seems to lie in the similarity of the names in Chinese.70 In another instance, a young woman suffering from lifelong eczema received an herbal cream from a Chinese practioner. She became suspicious after its effects resembled those of other corticosteroid creams she had used. She sent a sample to the Leicester Royal Infirmary for analysis and the presence of a corticosteroid, possibly fluocortolone or prednisolone, was confirmed.92 In yet another case, FDA researchers determined that a large batch of plant material laced with digitalis was sold to several herbal companies in the US. Digitalis can cause nausea, vomiting, and irregular heartbeats.73
Many studies call into question the purity and content of herbal products. Bahrke and Morgan55 reported on quantitative differences in individual and total ginsenosides within herbal products. The factors affecting these differences were species, growing environment, soil and fertility conditions, age of the roots, different parts of the plant, and extraction methods.98 These aforementioned factors may also play a role in the physiologic effects of ginseng, which might explain the reported adverse effects.55 Many of the problems associated with the adulteration, variable purity, and potency of herbs could be addressed with improved manufacturing and quality standards.
Product Manufacturing
The DSHEA granted authority to the FDA to establish “good manufacturing practices” for herbal products.6 These regulations would govern the preparation, packing, and holding of dietary supplements under conditions that assure their safety. These regulations are to be modeled under guidelines currently in effect for the food industry. To date, the FDA has not fully implemented manufacturing guidelines for the herbal industry.99
Good manufacturing practices to ensure purity and potency of products were a common theme during the June 1999 Dietary Supplement Stakeholder Meeting held by the FDA's Center for Food Safety and Applied Nutrition.99 This meeting included participants from every aspect of the herbal industry. At the center of the manufacturing discussion is the idea of standardization. Setting standards for supplements would mean that a specified amount of a herb is detectable, measurable, and known to have a biological response in the body.100 This desired consistency does not currently exist. Resolving this problem of standardizing and regulating herbal supplements is difficult. Differences in soil quality, percentage of herb utilized, harvest time, climate changes, growing seasons, and exposure to light are some factors that may affect herb quality.100 While the need to improve manufacturing practices is widely accepted, lack of agreement on standards and rules for enforcement has slowed the bureaucratic rule-making process.99
The herbal industry has taken strides to police itself with regard to product quality. The National Nutritional Foods Association randomly tests products produced by its members. The Association also plans to begin certification of factories every 3 years using the same good manufacturing processes proposed by the FDA, although manufacturers are not obligated to belong to this organization. In addition to the National Nutritional Foods Association, the United States Phamacopeia (USP) sets standards for pharmaceuticals, vitamins, and minerals. The USP, a private, nonprofit organization, has begun to produce monographs about herbs that sum up evidence of effectiveness and detail standards for quality, strength, and purity of the final product.1,99 Adoption of these standards is voluntary, and manufacturers claiming to meet them are not checked except in response to complaints.
CONCLUSIONS
Despite the increased tendency to seek natural therapies, athletes need to be aware that “natural” does not equal “safe.” Herbs should not be touted as miraculous cure-alls but rather compounds that work through simple biochemistry. Specific compounds trigger a specific physiologic effect—an effect that can be exacerbated if too much of a product is used or if it is used in combination with other medications. Athletes are entitled to know that most herbs are not proven safe and effective under current FDA standards. In addition, athletes may be unaware that the hyperbolic advertising and advocacy literature surrounding herbal products often contains untested claims. If an athlete wishes to take an herbal supplement, he or she should use a standardized product. Products should have the scientific name and quantity of the botanical clearly identified on the label. The name and address of the manufacturer, lot number, and expiration date should be clearly marked.4
Given the risks of toxicity and drug interaction, questions regarding the use of herbal supplements are essential when a health care provider takes a complete history. Athletes should consult a physician about potential drug interactions (both over the counter and prescription) before taking an herbal supplement. They should be advised to stop taking the herb immediately if adverse effects occur. Athletic trainers and physicians must be aware that herb use is deeply rooted in specific cultures and a key component of folk medicine. Therefore, an appropriate level of cultural sensitivity must be used when discussing the use of these products with athletes. Being judgmental or dismissive when discussing herbal products can erode the athlete's trust. The sports medicine team must be able to provide honest, unbiased information to educate athletes regarding herbal supplements.
ACKNOWLEDGMENTS
We thank Rick Mynark, PhD, and Rebecca Nelson, MS, ATC, for their assistance in the preparation of this manuscript.
REFERENCES
- 1.Herbal Rx: the promises and pitfalls. Consum Rep. 1999 Mar;:64. [PubMed] [Google Scholar]
- 2.Youngkin EQ, Israel DS. A review and critique of common herbal alternative therapies. Nurse Pract. 1999;21:39–52. doi: 10.1097/00006205-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 4.Tyler VE. What pharmacists should know about herbal remedies. J Am Pharm Assoc (Wash) 1996;NS36:29–37. doi: 10.1016/s1086-5802(16)30004-3. [DOI] [PubMed] [Google Scholar]
- 5.Eliason BC, Myszkowski J, Marbella A, Rasmann DN. Use of dietary supplements by patients in a family practice clinic. J Am Board Fam Pract. 1996;9:249–253. [PubMed] [Google Scholar]
- 6.Public Law 103-418 (S. 798) Dietary Supplement Health and Education Act of 1994. 1994. pp. 1–47. 103rd Cong, 2nd Sess.
- 7.Sobal J, Marquart LF. Vitamin/mineral supplement use among athletes: a review of the literature. Int J Sport Nutr. 1994;4:320–334. doi: 10.1123/ijsn.4.4.320. [DOI] [PubMed] [Google Scholar]
- 8.Washington, DC: US Food and Drug Administration; 1962. United States Code Title 21, Chapter 9, Section 360d Federal Food, Drug, and Cosmetic Act. 1962 Drug Amendments. [Google Scholar]
- 9.Tyler VE. The Honest Herbal. 3rd ed. New York, NY: Pharmaceutical Products Press; 1993. [Google Scholar]
- 10.Gruenwald J, Brendler T, Jaenicke C. Physician's Desk Reference for Herbal Medicines. 1st ed. Montvale, NJ: Medical Economics Co; 1998. p. 1244. [Google Scholar]
- 11.Herbal roulette. Consum Rep. 1995 Nov;:698–705. [Google Scholar]
- 12.Rockville, MD: FDA Center for Food Safety and Applied Nutrition; 1993. Illnesses and injuries associated with the use of selected dietary supplements. [Google Scholar]
- 13.Benzi G, Ceci A. Herbal medicines in European regulation. Pharmacol Res. 1997;35:355–362. doi: 10.1006/phrs.1997.0132. [DOI] [PubMed] [Google Scholar]
- 14.Gray MA. Herbs: multicultural folk medicines. Orthop Nurs. 1996;15:49–56. [PubMed] [Google Scholar]
- 15.Tyler V. Herbs of Choice: The Therapeutic Use of Phytomedicinals. New York, NY: Pharmaceutical Products Press; 1994. [Google Scholar]
- 16.Marwick C. Growing use of medicinal botanicals forces assessment by drug regulators. JAMA. 1995;273:607–609. [PubMed] [Google Scholar]
- 17.D'Arcy PF. Adverse reactions and interactions with herbal medicines, part 1: adverse reactions. Adverse Drug React Toxicol Rev. 1991;10:189–208. [PubMed] [Google Scholar]
- 18.Lin JL, Ho YS. Flavonoid-induced acute nephropathy. Am J Kidney Dis. 1994;23:433–440. doi: 10.1016/s0272-6386(12)81008-0. [DOI] [PubMed] [Google Scholar]
- 19.Anderson IB, Mullen WH, Meeker JE, et al. Pennyroyal toxicity: measurement of toxic metabolite levels in two cases and review of the literature. Ann Intern Med. 1996;124:726–734. doi: 10.7326/0003-4819-124-8-199604150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Larrey D, Vial T, Pauwels A, et al. Hepatitis after germander (Teucrium chamaedrys) administration: another instance of herbal medicine hepatotoxicity. Ann Intern Med. 1992;117:129–132. doi: 10.7326/0003-4819-117-2-129. [DOI] [PubMed] [Google Scholar]
- 21.Mostefa-Kara N, Pauwels A, Pines E, Biour M, Levy VG. Fatal hepatitis after herbal tea. Lancet. 1992;340:674. [PubMed] [Google Scholar]
- 22.Caldwell SH, Feeley JW, Wieboldt TF, Featherston PL, Dickson RC. Acute hepatitis with use of over-the-counter herbal remedies. Va Med Q. 1994;121:31–33. [PubMed] [Google Scholar]
- 23.Gordon DW, Rosenthal G, Hart J, Sirota R, Baker AL. Chaparral ingestion: the broadening spectrum of liver injury caused by herbal medications. JAMA. 1995;273:489–490. doi: 10.1001/jama.273.6.489. [DOI] [PubMed] [Google Scholar]
- 24.Miskelly FG, Goodyer LI. Hepatic and pulmonary complications of herbal medicine. Postgrad Med J. 1992;68:935. doi: 10.1136/pgmj.68.805.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai RS, Chiang AA, Wu MT, et al. Outbreak of bronchiolitis obliterans associated with consumption of Sauropus androgynus in Taiwan. Lancet. 1996;348:83–85. doi: 10.1016/s0140-6736(96)00450-3. [DOI] [PubMed] [Google Scholar]
- 26.Vanherweghem JL, Depierreux M, Tielemans C, et al. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 27.Jadoul M, de Plaen JF, Cosyns JP, Van Ypersele de Strihou C. Adverse effects from traditional Chinese medicine. Lancet. 1995;341:892–893. [PubMed] [Google Scholar]
- 28.Vanhaelen M, Vanhaelen-Fastre R, But P, Vanherweghem JL. Identification of aristolochic acid in Chinese herbs. Lancet. 1994;343:174. doi: 10.1016/s0140-6736(94)90964-4. [DOI] [PubMed] [Google Scholar]
- 29.Tomlinson B, Chan TY, Chan JC, Critchley JA. Herb-induced aconitine poisoning. Lancet. 1993;341:370–371. doi: 10.1016/0140-6736(93)90169-h. [DOI] [PubMed] [Google Scholar]
- 30.Tai YT, But PP, Young K, Lau CP. Adverse effects from traditional Chinese medicine. Lancet. 1993;341:892. [PubMed] [Google Scholar]
- 31.Woolf GM, Petrovic LM, Rojter SE, et al. Acute hepatitis associated with the Chinese herbal product jin bu huan. Ann Intern Med. 1994;121:729–735. doi: 10.7326/0003-4819-121-10-199411150-00001. [DOI] [PubMed] [Google Scholar]
- 32.Graham-Brown R. Toxicity of Chinese herbal remedies. Lancet. 1992;340:673–674. doi: 10.1016/0140-6736(92)92208-w. [DOI] [PubMed] [Google Scholar]
- 33.Perharic-Walton L, Murray L. Toxicity of Chinese herbal remedies. Lancet. 1992;340:674. [PubMed] [Google Scholar]
- 34.Kao WF, Hung DZ, Tsai WJ, Lin KP, Deng JF. Podophyllotoxin intoxication: toxic effect of Bajiaolian in herbal therapeutics. Hum Exp Toxicol. 1992;11:480–487. doi: 10.1177/096032719201100607. [DOI] [PubMed] [Google Scholar]
- 35.Itoh S, Marutani K, Nishijima T, Matsuo S, Itabashi M. Liver injuries induced by herbal medicine, syo-saiko-to (xiao-chai-hu-tang) Dig Dis Sci. 1995;40:1845–1848. doi: 10.1007/BF02212712. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:65–70. doi: 10.1111/j.1600-0536.1992.tb05211.x. [DOI] [PubMed] [Google Scholar]
- 37.Perron AD, Patterson JA, Yanofsky NN. Kumbucha “mushroom” hepatotoxicity. Ann Emerg Med. 1995;26:660–661. doi: 10.1016/s0196-0644(95)70028-5. [DOI] [PubMed] [Google Scholar]
- 38.Wilkie A, Cordess C. Ginseng--a root just like a carrot? J R Soc Med. 1994;87:594–595. doi: 10.1177/014107689408701009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McRae S. Elevated serum digoxin levels in a patient taking dogoxin and Siberian ginseng. CMAJ. 1996;155:293–295. [PMC free article] [PubMed] [Google Scholar]
- 40.Dega H, Laporte JL, Frances C, Herson S, Chosidow O. Ginseng as a cause of Stevens-Johnson syndrome? Lancet. 1996;347:1344. doi: 10.1016/s0140-6736(96)91001-6. [DOI] [PubMed] [Google Scholar]
- 41.Ryu SJ, Chien YY. Ginseng-associated cerebral arteritis. Neurology. 1995;45:829–830. doi: 10.1212/wnl.45.4.829. [DOI] [PubMed] [Google Scholar]
- 42.Doyle H, Kargin M. Herbal stimulant containing ephedrine has also caused psychosis. Br Med J. 1996;313:756. doi: 10.1136/bmj.313.7059.756b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cahill DJ, Fox R, Wardle PG, Harlow CR. Multiple follicular development associated with herbal medicine. Hum Reprod. 1994;9:1469–1470. doi: 10.1093/oxfordjournals.humrep.a138731. [DOI] [PubMed] [Google Scholar]
- 44.Lewis JH. Esophageal and small bowel obstruction from guar gum-containing “diet pills”: analysis of 26 cases reported to the Food and Drug Administration. Am J Gastroenterol. 1992;87:1424–1428. [PubMed] [Google Scholar]
- 45.Galloway JH, Farmer K, Weeks GR, Marsh ID, Forrest AR. Potentially hazardous compound in a herbal slimming remedy. Lancet. 1992;340:179. doi: 10.1016/0140-6736(92)93257-n. [DOI] [PubMed] [Google Scholar]
- 46.Greenwald J. Herbal healing. Time. 1998 Nov 23;:58–68. [Google Scholar]
- 47.Myers JB, Guskiewicz KM, Riemann BL. Syncope and atypical chest pain in an intercollegiate wrestler: a case report. J Athl Train. 1999;34:263–266. [PMC free article] [PubMed] [Google Scholar]
- 48.US Food and Drug Administration. Rockville, MD: National Press Office; 1996. FDA statement on street drugs containing botanical ephedrine. [Google Scholar]
- 49.Dietary supplements containing ephedrine alkaloids: proposed rule. Federal Register. 1997:30677–30724. [PubMed] [Google Scholar]
- 50.Dietary supplements containing ephedrine alkaloids: availability. Federal Register. 2000:17510–17512. [PubMed] [Google Scholar]
- 51.Lewis CJ. Rockville, MD: FDA Center for Food Safety and Applied Nutrition; 2000. Letter to industry: FDA concerned about botanical products, including dietary supplements containing aristolochic acid. [Google Scholar]
- 52.Are your chronically ill patients turning to herbs? Some cause potentially dangerous interactions. Disease State Management. 1999;5:66–70. [Google Scholar]
- 53.O'Neil CK, Avila JR, Fetrow CW. Herbal medicines: getting beyond the hype. Nursing99. 1999 Apr;:58–61. doi: 10.1097/00152193-199904000-00019. [DOI] [PubMed] [Google Scholar]
- 54.Scheller M. Herbal hope or herbal hype? Current Health 2. 1998;25:21. [Google Scholar]
- 55.Bahrke MS, Morgan WP. Evaluation of the ergogenic properties of ginseng. Sports Med. 1994;18:229–248. doi: 10.2165/00007256-199418040-00003. [DOI] [PubMed] [Google Scholar]
- 56.Siegel RK. Ginseng abuse syndrome: problems with the panacea. JAMA. 1979;241:1614–1615. [PubMed] [Google Scholar]
- 57.Siegel R. Ginseng and high blood pressure. JAMA. 1980;243:32. [PubMed] [Google Scholar]
- 58.Shaw D, Leon C, Kolev S, Murray V. Traditional remedies and food supplements: a 5-year toxicological study (1991–1995) Drug Saf. 1997;17:342–356. doi: 10.2165/00002018-199717050-00006. [DOI] [PubMed] [Google Scholar]
- 59.Gardiner P, Kemper KJ. Herbs in pediatric and adolescent medicine. Pediatr Rev. 2000;21:44–57. doi: 10.1542/pir.21-2-44. [DOI] [PubMed] [Google Scholar]
- 60.Heiligenstein E, Guenther G. Over-the-counter psychotropics: a review of melatonin, St. John's wort, valerian, and kava-kava. J Am Coll Health. 1998;46:271–276. doi: 10.1080/07448489809596003. [DOI] [PubMed] [Google Scholar]
- 61.Dorn M, Knick E, Lewith G. Placebo-controlled, double-blind study of Echinaceae pallidaeradix in upper respiratory tract infections. Complement Ther Med. 1997;5:40–42. [Google Scholar]
- 62.Melchart D, Walther E, Linde K, Brandmaier R, Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections: a double-blind, placebo-controlled randomized trial. Arch Fam Med. 1998;7:541–545. doi: 10.1001/archfami.7.6.541. [DOI] [PubMed] [Google Scholar]
- 63.Muirhead G. Herbal medicines you can recommend with confidence. Patient Care. 1999;33:76–94. [Google Scholar]
- 64.Raloff J. New support for echinacea's benefits. Science News. 1999;155:207. [Google Scholar]
- 65.Vogler BH, Pittler MH, Ernst E. Feverfew as a preventive treatment for migraine: a systematic review. Cephalalgia. 1998;18:704–708. doi: 10.1046/j.1468-2982.1998.1810704.x. [DOI] [PubMed] [Google Scholar]
- 66.Le Bars PL, Katz MM, Berman N, Itil TM, Freedman AM, Schatzberg AF. A placebo controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia: North American EGb Study Group. JAMA. 1997;278:1327–1332. doi: 10.1001/jama.278.16.1327. [DOI] [PubMed] [Google Scholar]
- 67.Schulz V, Hansel R, Tyler V. Rational Phytotherapy. 3rd ed. Berlin, Germany: Springer-Verlag; 1998. pp. 270–272. [Google Scholar]
- 68.Wilt T, Ishani A, Stark G. Saw palmetto extracts for treatment of benign prostatic hyperplasia: a systematic review. JAMA. 1998;280:1604–1609. doi: 10.1001/jama.280.18.1604. [DOI] [PubMed] [Google Scholar]
- 69.Fischman J. Herbs and prescriptions can make a risky mixture. US News & World Report. 2000 May 1;128:64–65. [PubMed] [Google Scholar]
- 70.Fugh-Bergman A. Herb-drug interactions. Lancet. 2000;355:134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 71.Arky R. Physician's Desk Reference. 53rd ed. Montvale, NJ: Medical Economics Co; 1999. [Google Scholar]
- 72.Heart information network. Center for Cardiovascular Education, Inc. Available at http://www.heartinfo.org/psyllium1296.htm. Accessed June 26, 2000.
- 73.Herb watch. Consum Rep Health. 1999;11:10. [Google Scholar]
- 74.But PP. Herbal poisoning caused by adulterants or erroneous substitutes. J Trop Med Hyg. 1994;97:371–374. [PubMed] [Google Scholar]
- 75.Awang D. Maternal use of ginseng and neonatal androgenization. JAMA. 1991;266:363. [PubMed] [Google Scholar]
- 76.Huang WF, Wen KC, Hsiao ML. Adulteration by synthetic therapeutic substances of traditional Chinese medicines in Taiwan. J Clin Pharmacol. 1997;37:344–350. doi: 10.1002/j.1552-4604.1997.tb04312.x. [DOI] [PubMed] [Google Scholar]
- 77.Gertner E, Marshall PS, Filandrinos D, Potek AS, Smith TM. Complications resulting from the use of Chinese herbal medications containing undeclared prescription drugs. Arthritis Rheum. 1995;38:614–617. doi: 10.1002/art.1780380506. [DOI] [PubMed] [Google Scholar]
- 78.Vander Stricht BI, Parvais OE, Vanhaelen-Fastre RJ, Vanhaelen MH, Quertinier D. Safer use of traditional remedies; remedies may contain cocktail of active drugs. Br Med J. 1994;308:1162. doi: 10.1136/bmj.308.6937.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keen RW, Deacon AC, Delves HT, Moreton JA, Frost PG. Indian herbal remedies for diabetes as a cause of lead poisoning. Postgrad Med J. 1994;70:113–114. doi: 10.1136/pgmj.70.820.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perharic L, Shaw D, Murray V. Toxic effects of herbal medicines and food supplements. Lancet. 1993;342:180–181. doi: 10.1016/0140-6736(93)91389-4. [DOI] [PubMed] [Google Scholar]
- 81.Dunbabin DA, Tallis GA, Popplewell PY, Lee RA. Lead poisoning from Indian herbal medicine (Ayurveda) Med J Aust. 1992;157:835–836. doi: 10.5694/j.1326-5377.1992.tb141305.x. [DOI] [PubMed] [Google Scholar]
- 82.Thatte UM, Rege NN, Phatak SD, Dahanukar SA. The flip side of Ayurveda. J Postgrad Med. 1993;39:179–182. [PubMed] [Google Scholar]
- 83.Bayly GR, Braithwaite RA, Sheehan TM, Dyer NH, Grimley C, Ferner RE. Lead poisoning from Asian traditional remedies in the West Midlands: report of a series of five cases. Hum Exp Toxicol. 1995;14:24–28. doi: 10.1177/096032719501400106. [DOI] [PubMed] [Google Scholar]
- 84.Kew J, Morris C, Aihic A, Fysh R, Jones S, Brooks D. Arsenic and mercury intoxication due to Indian ethnic remedies. Br Med J. 1993;306:506–507. doi: 10.1136/bmj.306.6876.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sheerin NS, Monk PN, Aslam M, Thurston H. Simultaneous exposure to lead, arsenic and mercury from Indian ethnic remedies. Br J Clin Pract. 1994;48:332–333. [PubMed] [Google Scholar]
- 86.Kshirsagar NA. Misleading herbal Ayurvedic brand name. Lancet. 1993;341:1595–1596. doi: 10.1016/0140-6736(93)90733-w. [DOI] [PubMed] [Google Scholar]
- 87.Capobianco DJ, Brazis PW, Fox TP. Proximal-muscle weakness induced by herbs. N Engl J Med. 1993;329:1430. doi: 10.1056/NEJM199311043291919. [DOI] [PubMed] [Google Scholar]
- 88.Diamond JR, Pallone TL. Acute interstitial nephritis following use of tung shueh pills. Am J Kidney Dis. 1994;24:219–221. doi: 10.1016/s0272-6386(12)80186-7. [DOI] [PubMed] [Google Scholar]
- 89.Abt AB, Oh JY, Huntington RA, Burkhart KK. Chinese herbal medicine induce acute renal failure. Arch Intern Med. 1995;155:211–212. [PubMed] [Google Scholar]
- 90.Hughes JR, Higgins EM, Pembroke AC. Oral dexamethasone masquerading as a Chinese herbal remedy. Br J Dermatol. 1994;130:261. doi: 10.1111/j.1365-2133.1994.tb02916.x. [DOI] [PubMed] [Google Scholar]
- 91.O'Driscoll J, Burden AD, Kingston TP. Potent topical steroid obtained from a Chinese herbalist. Br J Dermatol. 1992;127:543–544. doi: 10.1111/j.1365-2133.1992.tb14859.x. [DOI] [PubMed] [Google Scholar]
- 92.Graham-Brown RA, Bourke JF, Bumphrey G. Chinese herbal remedies may contain steroids. Br Med J. 1994;308:473. doi: 10.1136/bmj.308.6926.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Markowitz SB, Nunez CM, Klitzman S, et al. Lead poisoning due to hai ge fen: the porphyrin content of individual erythrocytes. JAMA. 1994;271:932–934. [PubMed] [Google Scholar]
- 94.Kang-Yum E, Oransky SH. Chinese patent medicine as a potential source of mercury poisoning. Vet Hum Toxicol. 1992;34:235–238. [PubMed] [Google Scholar]
- 95.Schaumburg HH, Berger A. Alopecia and sensory polyneuropathy from thallium in a Chinese herbal medication. JAMA. 1992;268:3430–3431. [PubMed] [Google Scholar]
- 96.Espinoza EO, Mann MJ, Bleasdell B. Arsenic and mercury in traditional Chinese herbal balls. N Engl J Med. 1995;333:803–804. doi: 10.1056/NEJM199509213331217. [DOI] [PubMed] [Google Scholar]
- 97.Webb D. Supplement news. Prevention. 2000;52:51. [Google Scholar]
- 98.Bahrke MS, Morgan WP. Evaluation of the ergogenic properties of ginseng: an update. Sports Med. 2000;29:113–133. doi: 10.2165/00007256-200029020-00004. [DOI] [PubMed] [Google Scholar]
- 99.Washington, DC: US Food and Drug Administration; 1999. Jun 8, Dietary Supplement Stakeholder Meeting. [Google Scholar]
- 100.Nelson Myer S, Novack J, Puntam K. Safety of herbal supplements. Top Clin Nutr. 1998;14:42–51. [Google Scholar]


