Abstract
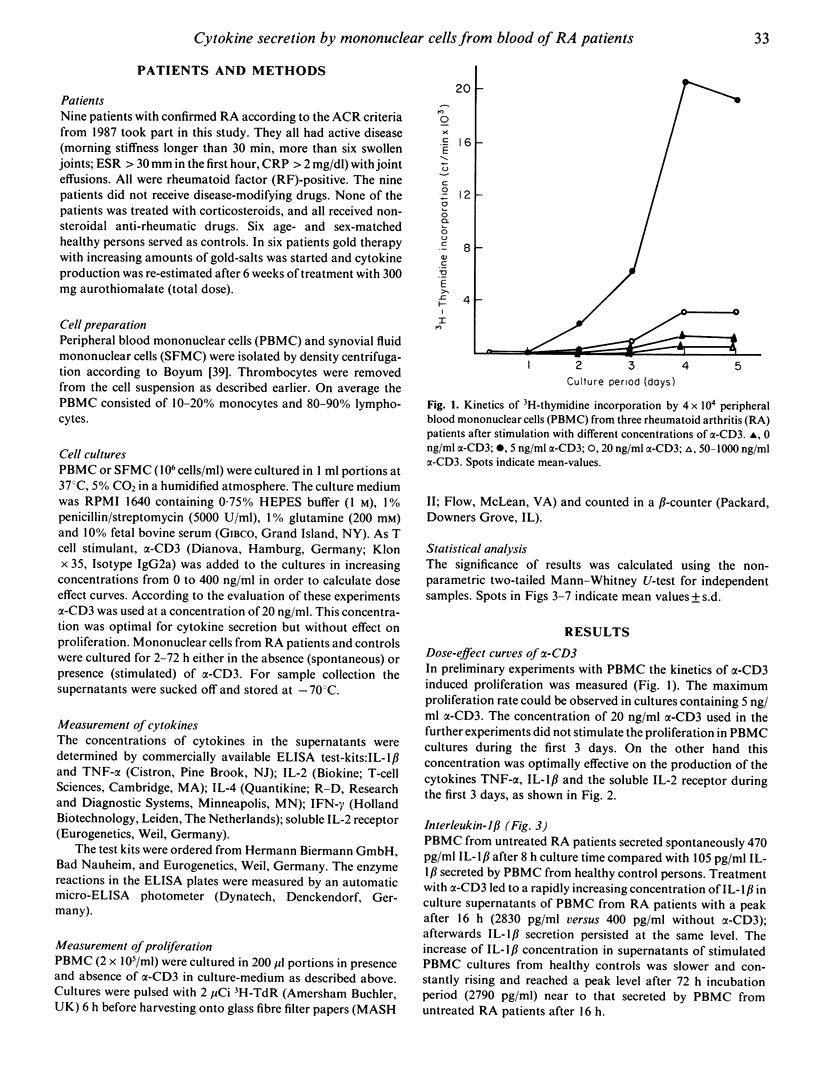
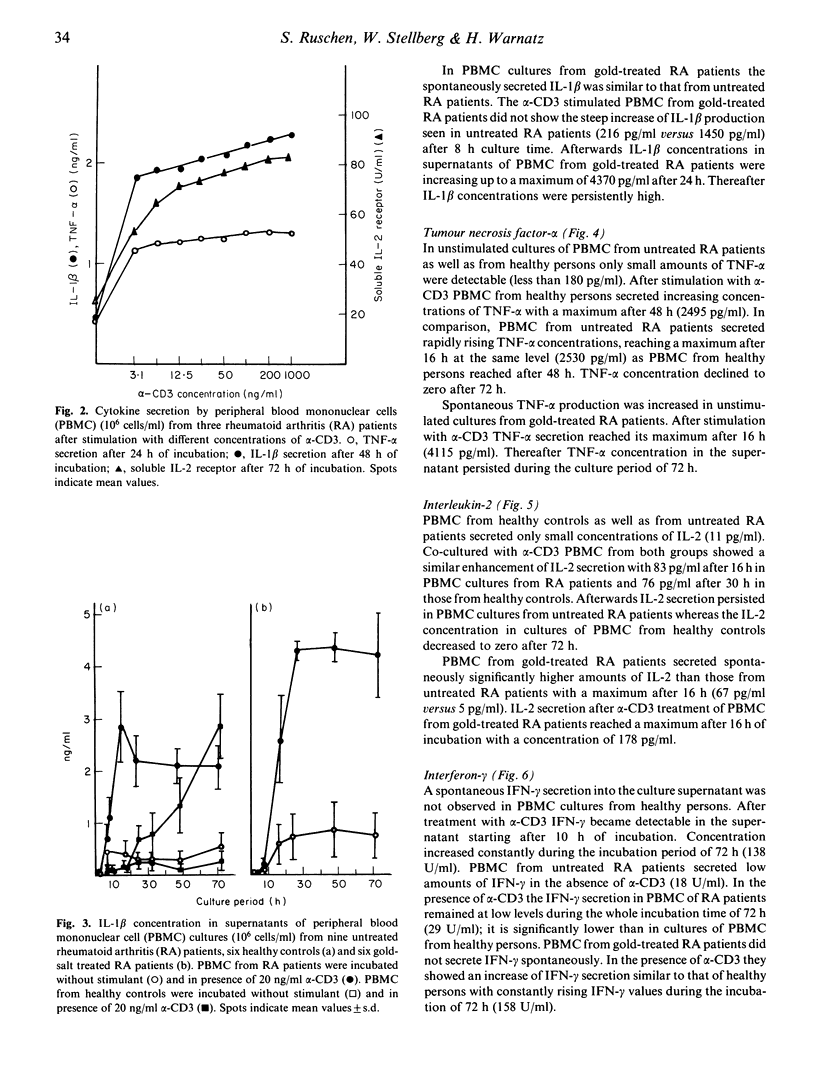
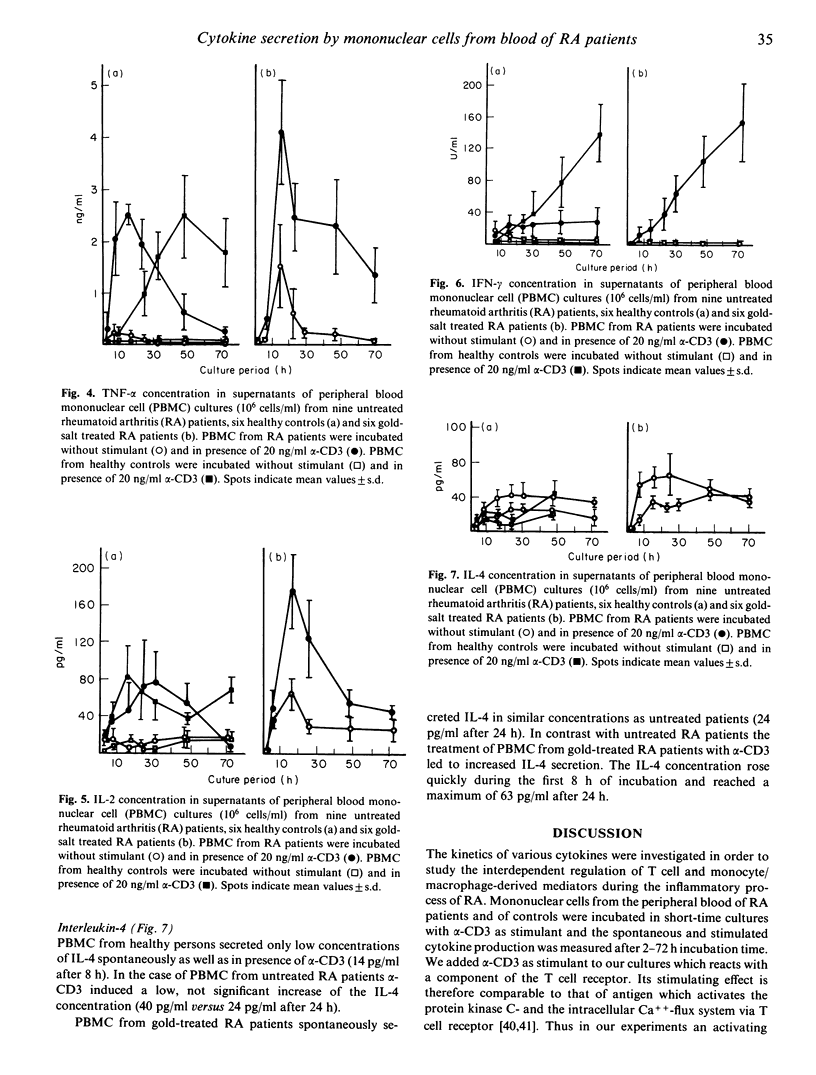
Mononuclear cells from peripheral blood (PBMC) of rheumatoid arthritis (RA) patients and healthy controls were incubated with alpha-CD3. Cytokine secretion from 2 h to 72 h of incubation was measured by ELISA. There were no significant differences in secretion of T cell derived IL-2 and IL-4 in cultures from RA patients and controls. The macrophage-derived cytokines, IL-1 beta and tumour-necrosis factor-alpha (TNF-alpha) were secreted with a steep increase of concentration during the first 16 h of incubation by PBMC from RA patients. PBMC from healthy controls secreted both cytokines at a constantly rising rate with a maximum for TNF-alpha at 48 h and for IL-1 beta at 72 h. Interferon-gamma (IFN-gamma) is secreted in significantly reduced concentrations by PBMC from untreated RA patients compared with controls. Gold-salt treatment led to a slightly delayed and enhanced secretion of TNF-alpha and IL-1 beta, an enhanced secretion of IL-2 and a restored secretion of IFN-gamma.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Dayer J. M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Joslin F. G., Thompson R. C., Hannum C. H. An IL-1 inhibitor from human monocytes. Production and characterization of biologic properties. J Immunol. 1989 Sep 15;143(6):1851–1858. [PubMed] [Google Scholar]
- Bhardwaj N., Lau L. L., Rivelis M., Steinman R. M. Interleukin-1 production by mononuclear cells from rheumatoid synovial effusions. Cell Immunol. 1988 Jul;114(2):405–423. doi: 10.1016/0008-8749(88)90332-2. [DOI] [PubMed] [Google Scholar]
- Bradley L. M., Duncan D. D., Tonkonogy S., Swain S. L. Characterization of antigen-specific CD4+ effector T cells in vivo: immunization results in a transient population of MEL-14-, CD45RB- helper cells that secretes interleukin 2 (IL-2), IL-3, IL-4, and interferon gamma. J Exp Med. 1991 Sep 1;174(3):547–559. doi: 10.1084/jem.174.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan F. M., Chantry D., Turner M., Foxwell B., Maini R., Feldmann M. Detection of transforming growth factor-beta in rheumatoid arthritis synovial tissue: lack of effect on spontaneous cytokine production in joint cell cultures. Clin Exp Immunol. 1990 Aug;81(2):278–285. doi: 10.1111/j.1365-2249.1990.tb03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan G., Barrett K., Turner M., Chantry D., Maini R. N., Feldmann M. Interleukin-1 and tumour necrosis factor mRNA expression in rheumatoid arthritis: prolonged production of IL-1 alpha. Clin Exp Immunol. 1988 Sep;73(3):449–455. [PMC free article] [PubMed] [Google Scholar]
- Chin J. E., Winterrowd G. E., Krzesicki R. F., Sanders M. E. Role of cytokines in inflammatory synovitis. The coordinate regulation of intercellular adhesion molecule 1 and HLA class I and class II antigens in rheumatoid synovial fibroblasts. Arthritis Rheum. 1990 Dec;33(12):1776–1786. doi: 10.1002/art.1780331204. [DOI] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Combe B., Pope R. M., Fischbach M., Darnell B., Baron S., Talal N. Interleukin-2 in rheumatoid arthritis: production of and response to interleukin-2 in rheumatoid synovial fluid, synovial tissue and peripheral blood. Clin Exp Immunol. 1985 Mar;59(3):520–528. [PMC free article] [PubMed] [Google Scholar]
- Czarniecki C. W., Chiu H. H., Wong G. H., McCabe S. M., Palladino M. A. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988 Jun 15;140(12):4217–4223. [PubMed] [Google Scholar]
- Dinarello C. A., Ikejima T., Warner S. J., Orencole S. F., Lonnemann G., Cannon J. G., Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987 Sep 15;139(6):1902–1910. [PubMed] [Google Scholar]
- Eastgate J. A., Symons J. A., Wood N. C., Grinlinton F. M., di Giovine F. S., Duff G. W. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988 Sep 24;2(8613):706–709. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Egeland T., Lund H. Immunoregulatory lymphokines in rheumatoid joints. I. Search for interleukin 2 in synovial fluid. Scand J Immunol. 1987 Jan;25(1):101–106. doi: 10.1111/j.1365-3083.1987.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Fava R., Olsen N., Keski-Oja J., Moses H., Pincus T. Active and latent forms of transforming growth factor beta activity in synovial effusions. J Exp Med. 1989 Jan 1;169(1):291–296. doi: 10.1084/jem.169.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Chantry D., Haworth C., Turner M., Abney E., Buchan G., Barrett K., Barkley D., Chu A. Cytokine production in the rheumatoid joint: implications for treatment. Ann Rheum Dis. 1990 Jun;49 (Suppl 1):480–486. [PubMed] [Google Scholar]
- Firestein G. S., Xu W. D., Townsend K., Broide D., Alvaro-Gracia J., Glasebrook A., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J Exp Med. 1988 Nov 1;168(5):1573–1586. doi: 10.1084/jem.168.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that gamma-interferon is not the primary macrophage activating factor. Arthritis Rheum. 1987 Aug;30(8):864–871. doi: 10.1002/art.1780300804. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. IV. Murine CTL clones produce IL-3 and GM-CSF, the activity of which is masked by the inhibitory action of secreted IFN-gamma. J Immunol. 1990 Jan 15;144(2):548–556. [PubMed] [Google Scholar]
- Guerne P. A., Zuraw B. L., Vaughan J. H., Carson D. A., Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989 Feb;83(2):585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Rowe F. M., Bird C. R., Gearing A. J. Production of interleukin 1 in the joint during the development of antigen-induced arthritis in the rabbit. Clin Exp Immunol. 1988 Dec;74(3):371–376. [PMC free article] [PubMed] [Google Scholar]
- Herrmann F., Cannistra S. A., Lindemann A., Blohm D., Rambaldi A., Mertelsmann R. H., Griffin J. D. Functional consequences of monocyte IL-2 receptor expression. Induction of IL-1 beta secretion by IFN gamma and IL-2. J Immunol. 1989 Jan 1;142(1):139–143. [PubMed] [Google Scholar]
- Hopkins S. J., Humphreys M., Jayson M. I. Cytokines in synovial fluid. I. The presence of biologically active and immunoreactive IL-1. Clin Exp Immunol. 1988 Jun;72(3):422–427. [PMC free article] [PubMed] [Google Scholar]
- Hopkins S. J., Meager A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin Exp Immunol. 1988 Jul;73(1):88–92. [PMC free article] [PubMed] [Google Scholar]
- Husby G., Williams R. C., Jr Synovial localization of tumor necrosis factor in patients with rheumatoid arthritis. J Autoimmun. 1988 Aug;1(4):363–371. doi: 10.1016/0896-8411(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Jayaram Y., Buckle A. M., Hogg N. The Fc receptor, FcRI, and other activation molecules on human mononuclear phagocytes after treatment with interferon-gamma. Clin Exp Immunol. 1989 Mar;75(3):414–420. [PMC free article] [PubMed] [Google Scholar]
- Kingsley G., Pitzalis C., Panayi G. S. Immunogenetic and cellular immune mechanisms in rheumatoid arthritis: relevance to new therapeutic strategies. Br J Rheumatol. 1990 Feb;29(1):58–64. doi: 10.1093/rheumatology/29.1.58. [DOI] [PubMed] [Google Scholar]
- Kumkumian G. K., Lafyatis R., Remmers E. F., Case J. P., Kim S. J., Wilder R. L. Platelet-derived growth factor and IL-1 interactions in rheumatoid arthritis. Regulation of synoviocyte proliferation, prostaglandin production, and collagenase transcription. J Immunol. 1989 Aug 1;143(3):833–837. [PubMed] [Google Scholar]
- Larrick J. W. Native interleukin 1 inhibitors. Immunol Today. 1989 Feb;10(2):61–66. doi: 10.1016/0167-5699(89)90308-3. [DOI] [PubMed] [Google Scholar]
- Lemm G., Warnatz H. Enumeration of T cell subsets and functional suppressor cell assays in patients with rheumatoid arthritis and HLA-B27-associated arthritis. Z Rheumatol. 1984 May-Jun;43(3):106–112. [PubMed] [Google Scholar]
- Lemm G., Warnatz H. Evidence for enhanced interleukin 2 (IL-2) secretion and IL-2 receptor presentation by synovial fluid lymphocytes in rheumatoid arthritis. Clin Exp Immunol. 1986 Apr;64(1):71–79. [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Kekow J., Carson D. A. Transforming growth factor-beta and cellular immune responses in synovial fluids. J Immunol. 1990 Jun 1;144(11):4189–4194. [PubMed] [Google Scholar]
- Miossec P., Naviliat M., Dupuy d'Angeac A., Sany J., Banchereau J. Low levels of interleukin-4 and high levels of transforming growth factor beta in rheumatoid synovitis. Arthritis Rheum. 1990 Aug;33(8):1180–1187. doi: 10.1002/art.1780330819. [DOI] [PubMed] [Google Scholar]
- Miossec P. The role of interleukin 1 in the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 1987 Oct-Dec;5(4):305–308. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nouri A. M., Panayi G. S., Goodman S. M. Cytokines and the chronic inflammation of rheumatic disease. II. The presence of interleukin-2 in synovial fluids. Clin Exp Immunol. 1984 Nov;58(2):402–409. [PMC free article] [PubMed] [Google Scholar]
- Ridley M. G., Panayi G. S., Nicholas N. S., Murphy J. Mechanisms of macrophage activation in rheumatoid arthritis: the role of gamma-interferon. Clin Exp Immunol. 1986 Mar;63(3):587–593. [PMC free article] [PubMed] [Google Scholar]
- Ruschen S., Lemm G., Warnatz H. Interleukin-2 secretion by synovial fluid lymphocytes in rheumatoid arthritis. Br J Rheumatol. 1988 Oct;27(5):350–356. doi: 10.1093/rheumatology/27.5.350. [DOI] [PubMed] [Google Scholar]
- Ruschen S., Lemm G., Warnatz H. Spontaneous and LPS-stimulated production of intracellular IL-1 beta by synovial macrophages in rheumatoid arthritis is inhibited by IFN-gamma. Clin Exp Immunol. 1989 May;76(2):246–251. [PMC free article] [PubMed] [Google Scholar]
- Sadouk M., Vaquero C., de la Tour B., Amor B., Toubert A. Interferon-gamma mRNA expression upon in vitro T lymphocyte activation is decreased in rheumatoid arthritis patients. Clin Immunol Immunopathol. 1990 Jul;56(1):37–45. doi: 10.1016/0090-1229(90)90167-o. [DOI] [PubMed] [Google Scholar]
- Schindler R., Ghezzi P., Dinarello C. A. IL-1 induces IL-1. IV. IFN-gamma suppresses IL-1 but not lipopolysaccharide-induced transcription of IL-1. J Immunol. 1990 Mar 15;144(6):2216–2222. [PubMed] [Google Scholar]
- Seckinger P., Williamson K., Balavoine J. F., Mach B., Mazzei G., Shaw A., Dayer J. M. A urine inhibitor of interleukin 1 activity affects both interleukin 1 alpha and 1 beta but not tumor necrosis factor alpha. J Immunol. 1987 Sep 1;139(5):1541–1545. [PubMed] [Google Scholar]
- Seitz M., Dewald B., Gerber N., Baggiolini M. Enhanced production of neutrophil-activating peptide-1/interleukin-8 in rheumatoid arthritis. J Clin Invest. 1991 Feb;87(2):463–469. doi: 10.1172/JCI115018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore A., Jaglal S., Keystone E. C. Enhanced interleukin 1 generation by monocytes in vitro is temporally linked to an early event in the onset or exacerbation of rheumatoid arthritis. Clin Exp Immunol. 1986 Aug;65(2):293–302. [PMC free article] [PubMed] [Google Scholar]
- Stekman I. L., Blasini A. M., Leon-Ponte M., Baroja M. L., Abadi I., Rodriguez M. A. Enhanced CD3-mediated T lymphocyte proliferation in patients with systemic lupus erythematosus. Arthritis Rheum. 1991 Apr;34(4):459–467. doi: 10.1002/art.1780340411. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wong H. L., Dougherty S., McCartney-Francis N., Wahl L. M., Ellingsworth L., Schmidt J. A., Hall G., Roberts A. B. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988 May 1;140(9):3026–3032. [PubMed] [Google Scholar]
- Warnatz H., Ruschen S., Lemm G. Interleukin-1 in der Pathogenese der chronischen Polyarthritis. Med Klin (Munich) 1990 May 15;85(5):302–307. [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]
- Yamagata N., Kobayashi K., Kasama T., Fukushima T., Tabata M., Yoneya I., Shikama Y., Kaga S., Hashimoto M., Yoshida K. Multiple cytokine activities and loss of interleukin 2 inhibitor in synovial fluids of patients with rheumatoid arthritis. J Rheumatol. 1988 Nov;15(11):1623–1627. [PubMed] [Google Scholar]
- Yocum D. E., Esparza L., Dubry S., Benjamin J. B., Volz R., Scuderi P. Characteristics of tumor necrosis factor production in rheumatoid arthritis. Cell Immunol. 1989 Aug;122(1):131–145. doi: 10.1016/0008-8749(89)90154-8. [DOI] [PubMed] [Google Scholar]


