Abstract
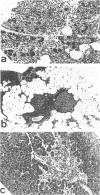
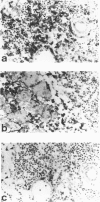
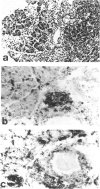
MRL/Mp mice are known to have autoimmune disease-prone genetic background, which contributes to the development of a lethal autoimmune disease at an early age in association with the lymphoproliferative gene, lpr. In this study, we found that MRL/Mp mice, not bearing lpr (MRL/Mp-(+)/+), spontaneously developed pancreatitis at a late stage of life, which was histopathologically characterized by destruction of pancreatic acinar cells with mononuclear cell infiltration. In female 34-38-weeks-old mice the incidence of pancreatitis reached 74%, whereas the male mice developed the disease with a reduced incidence, at a later stage of life and with a reduced severity. Cell infiltrates in the affected lesions were composed predominantly of CD4+ cells and to lesser extent Mac-2+ macrophages. Adoptive transfer of the spleen cells obtained from pancreatitis-bearing female mice generated pancreatitis in female normal mice, but not in the male mice. Transfer of the serum of pancreatitis-bearing mice failed to induce any pancreatic lesions. These findings indicate that pancreatitis in MRL/Mp-(+)/+ mice may be mediated by cellular autoimmune mechanism. This may present a useful concept for analysis of the developmental mechanisms of human chronic pancreatitis in an aspect of autoimmunity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. J., Dyer P. A., Donnai D., Klouda P. T., Jennison R., Braganza J. M. Chronic pancreatitis, HLA and autoimmunity. Int J Pancreatol. 1988 Jan-Feb;3(1):83–90. doi: 10.1007/BF02788226. [DOI] [PubMed] [Google Scholar]
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedossa P., Bacci J., Lemaigre G., Martin E. Lymphocyte subsets and HLA-DR expression in normal pancreas and chronic pancreatitis. Pancreas. 1990 Jul;5(4):415–420. doi: 10.1097/00006676-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Brick J. E., Walker S. E., Wise K. S. Hormone control of autoantibodies to calf thymus nuclear extract (CTE) and DNA in MRL-lpr and MRL-+/+ mice. Clin Immunol Immunopathol. 1988 Jan;46(1):68–81. doi: 10.1016/0090-1229(88)90007-4. [DOI] [PubMed] [Google Scholar]
- Dang-Vu A. P., Pisetsky D. S., Weinberg J. B. Functional alterations of macrophages in autoimmune MRL-lpr/lpr mice. J Immunol. 1987 Mar 15;138(6):1757–1761. [PubMed] [Google Scholar]
- Forbes A., Schwarz G., Mirakian R., Drummond V., Chan C. K., Cotton P. B., Bottazzo G. F. HLA antigens in chronic pancreatitis. Tissue Antigens. 1987 Oct;30(4):176–183. doi: 10.1111/j.1399-0039.1987.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Hart M. N., Tassell S. K., Sadewasser K. L., Schelper R. L., Moore S. A. Autoimmune vasculitis resulting from in vitro immunization of lymphocytes to smooth muscle. Am J Pathol. 1985 Jun;119(3):448–455. [PMC free article] [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982 Mar;128(3):1221–1228. [PubMed] [Google Scholar]
- Hoffman R. W., Alspaugh M. A., Waggie K. S., Durham J. B., Walker S. E. Sjögren's syndrome in MRL/l and MRL/n mice. Arthritis Rheum. 1984 Feb;27(2):157–165. doi: 10.1002/art.1780270206. [DOI] [PubMed] [Google Scholar]
- Jones F. S., Pisetsky D. S., Kurlander R. J. Defects in mononuclear phagocytic system (MPS) function in autoimmune MRL-lpr/lpr mice. Clin Immunol Immunopathol. 1985 Jul;36(1):30–39. doi: 10.1016/0090-1229(85)90036-4. [DOI] [PubMed] [Google Scholar]
- Lankisch P. G., Koop H., Seelig R., Seelig H. P. Antinuclear and pancreatic acinar cell antibodies in pancreatic diseases. Digestion. 1981;21(2):65–68. doi: 10.1159/000198543. [DOI] [PubMed] [Google Scholar]
- Lendrum R., Walker G. Serum antibodies in human pancreatic disease. Gut. 1975 May;16(5):365–371. [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Nose M., Katoh M., Okada N., Kyogoku M., Okada H. Tissue distribution of HRF20, a novel factor preventing the membrane attack of homologous complement, and its predominant expression on endothelial cells in vivo. Immunology. 1990 Jun;70(2):145–149. [PMC free article] [PubMed] [Google Scholar]
- Nose M., Nishimura M., Kyogoku M. Analysis of granulomatous arteritis in MRL/Mp autoimmune disease mice bearing lymphoproliferative genes. The use of mouse genetics to dissociate the development of arteritis and glomerulonephritis. Am J Pathol. 1989 Aug;135(2):271–280. [PMC free article] [PubMed] [Google Scholar]
- Rumessen J. J., Marner B., Pedersen N. T., Permin H. Autoantibodies in chronic pancreatitis. Scand J Gastroenterol. 1985 Oct;20(8):966–970. doi: 10.3109/00365528509088856. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Roths J. B., Murphy E. D., Steinberg R. T., Raveche E. S. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J Immunol. 1980 Aug;125(2):871–873. [PubMed] [Google Scholar]