Abstract
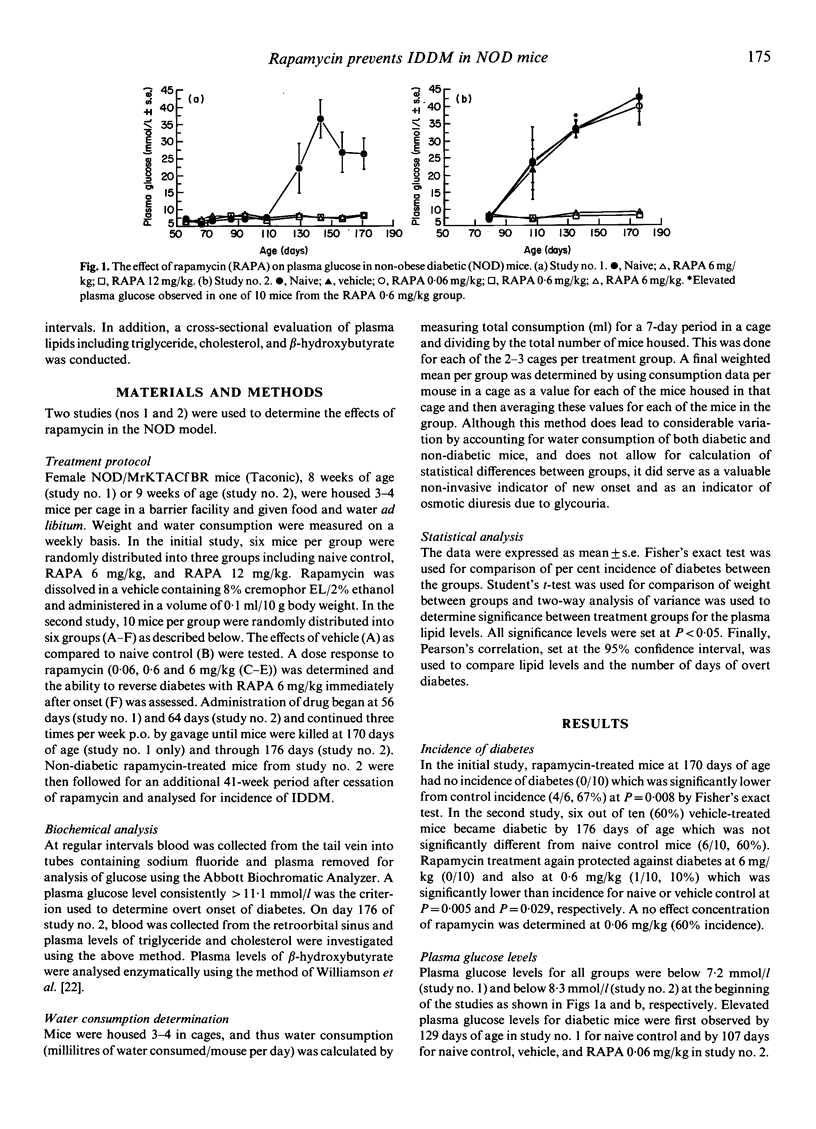
The effect of the immunosuppressive agent rapamycin (RAPA) was assessed in the non-obese diabetic (NOD) mouse which is an autoimmune model of IDDM. RAPA was prepared in a vehicle of 8% cremophor EL/2% ethanol and investigated in two studies. NOD/MrK female mice (six per group, study no. 1; 10 per group, study no. 2) were dosed three times per week p.o. by gavage from 56 to 170 days of age (study no. 1) or from 64 to 176 days of age (study no. 2). Mice treated with RAPA at 0.6 mg/kg, 6 mg/kg, or 12 mg/kg maintained normal plasma glucose through 170 or 176 days of age with 10%, 0%, and 0% incidence of diabetes respectively. In contrast, naive, vehicle-treated, or RAPA 0.06 mg/kg-treated mice exhibited elevated plasma glucose and disease incidence typical for female NOD mice. Mice which became diabetic had elevated levels of beta-hydroxybutyrate, triglycerides and cholesterol. These plasma lipid concentrations were positively correlated with the duration of hyperglycaemia (r = 0.85, 0.87 and 0.84 respectively). Outside of its ability to prevent diabetes, RAPA itself did not affect the lipid profile of the mice. Intervention therapy with RAPA was ineffective at reversing the course of disease after IDDM onset under these experimental conditions. Finally, we report here that prophylactic treatment with RAPA was able to protect against IDDM development in some RAPA-treated mice 41 weeks after cessation of treatment. These data show that orally administered RAPA is effective in preventing onset of disease in the NOD mouse, a relevant model of autoimmune type I diabetes in man.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., McDevitt H. O. The first external domain of the nonobese diabetic mouse class II I-A beta chain is unique. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2435–2439. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. A., Maclaren N. K., Luchetta R. Insulitis and diabetes in NOD mice reduced by prophylactic insulin therapy. Diabetes. 1990 Aug;39(8):933–937. doi: 10.2337/diab.39.8.933. [DOI] [PubMed] [Google Scholar]
- Atkinson M. A., Maclaren N. K. What causes diabetes? Sci Am. 1990 Jul;263(1):62-3, 66-71. doi: 10.1038/scientificamerican0790-62. [DOI] [PubMed] [Google Scholar]
- Calne R. Y., Collier D. S., Lim S., Pollard S. G., Samaan A., White D. J., Thiru S. Rapamycin for immunosuppression in organ allografting. Lancet. 1989 Jul 22;2(8656):227–227. doi: 10.1016/s0140-6736(89)90417-0. [DOI] [PubMed] [Google Scholar]
- Collier D. S., Calne R., Thiru S., Lim S., Pollard S. G., Barron P., Da Costa M., White D. J. Rapamycin in experimental renal allografts in dogs and pigs. Transplant Proc. 1990 Aug;22(4):1674–1675. [PubMed] [Google Scholar]
- Dumont F. J., Staruch M. J., Koprak S. L., Melino M. R., Sigal N. H. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990 Jan 1;144(1):251–258. [PubMed] [Google Scholar]
- Eng C. P., Gullo-Brown J., Chang J. Y., Sehgal S. N. Inhibition of skin graft rejection in mice by rapamycin: a novel immunosuppressive macrolide. Transplant Proc. 1991 Feb;23(1 Pt 1):868–869. [PubMed] [Google Scholar]
- Formby B., Miller N., Garret R., Peterson C. M. Effects of low-dose cyclosporine prophylaxis in nonobese diabetic mice. J Pharmacol Exp Ther. 1987 Jun;241(3):1106–1111. [PubMed] [Google Scholar]
- Fulop M., Eder H. A. Plasma triglycerides and cholesterol in diabetic ketosis. Arch Intern Med. 1989 Sep;149(9):1997–2002. [PubMed] [Google Scholar]
- HARRIS L. V. D., ALBRINK M. J., VAN ECK W. F., MAN E. B., PETERS J. P. Serum lipids in diabetic acidosis. Metabolism. 1953 Mar;2(2):120–132. [PubMed] [Google Scholar]
- Hattori M., Buse J. B., Jackson R. A., Glimcher L., Dorf M. E., Minami M., Makino S., Moriwaki K., Kuzuya H., Imura H. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986 Feb 14;231(4739):733–735. doi: 10.1126/science.3003909. [DOI] [PubMed] [Google Scholar]
- Kahan B. D., Chang J. Y., Sehgal S. N. Preclinical evaluation of a new potent immunosuppressive agent, rapamycin. Transplantation. 1991 Aug;52(2):185–191. doi: 10.1097/00007890-199108000-00001. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Holbeck S. L., Nisperos B., Hansen J. A., Maeda H., Nepom G. T. Identification of a polymorphic variant associated with HLA-DQw3 and characterized by specific restriction sites within the DQ beta-chain gene. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8139–8143. doi: 10.1073/pnas.82.23.8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurasawa K., Koike T., Matsumura R., Takabayashi K., Tomioka H., Ito I., Yoshida S. The immunosuppressant FK-506 prevents progression of diabetes in nonobese diabetic mice. Clin Immunol Immunopathol. 1990 Nov;57(2):274–279. doi: 10.1016/0090-1229(90)90041-n. [DOI] [PubMed] [Google Scholar]
- Laupacis A., Stiller C. R., Gardell C., Keown P., Dupre J., Wallace A. C., Thibert P. Cyclosporin prevents diabetes in BB Wistar rats. Lancet. 1983 Jan 1;1(8314-5):10–12. doi: 10.1016/s0140-6736(83)91558-1. [DOI] [PubMed] [Google Scholar]
- Maclaren N. K. How, when, and why to predict IDDM. Diabetes. 1988 Dec;37(12):1591–1594. doi: 10.2337/diab.37.12.1591. [DOI] [PubMed] [Google Scholar]
- Morel P. A., Dorman J. S., Todd J. A., McDevitt H. O., Trucco M. Aspartic acid at position 57 of the HLA-DQ beta chain protects against type I diabetes: a family study. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8111–8115. doi: 10.1073/pnas.85.21.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y., Suko M., Okudaira H., Matsuba I., Tsuruoka A., Sasaki A., Yokoyama H., Tanase T., Shida T., Nishimura M. Preventive effects of cyclosporin on diabetes in NOD mice. Diabetologia. 1986 Apr;29(4):244–247. doi: 10.1007/BF00454884. [DOI] [PubMed] [Google Scholar]
- Morris R. E., Meiser B. M., Wu J., Shorthouse R., Wang J. Use of rapamycin for the suppression of alloimmune reactions in vivo: schedule dependence, tolerance induction, synergy with cyclosporine and FK 506, and effect on host-versus-graft and graft-versus-host reactions. Transplant Proc. 1991 Feb;23(1 Pt 1):521–524. [PubMed] [Google Scholar]
- Morris R. E. Rapamycin: FK506's fraternal twin or distant cousin? Immunol Today. 1991 May;12(5):137–140. doi: 10.1016/S0167-5699(05)80040-4. [DOI] [PubMed] [Google Scholar]
- Murase N., Lieberman I., Nalesnik M. A., Mintz D. H., Todo S., Drash A. L., Starzl T. E. Effect of FK 506 on spontaneous diabetes in BB rats. Diabetes. 1990 Dec;39(12):1584–1586. doi: 10.2337/diab.39.12.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto H., Kikutani H., Yamamura K., Kishimoto T. Prevention of autoimmune insulitis by expression of I-E molecules in NOD mice. 1987 Jul 30-Aug 5Nature. 328(6129):432–434. doi: 10.1038/328432a0. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H., Worthen S. M., Shultz L. D. NOD marrow stem cells adoptively transfer diabetes to resistant (NOD x NON)F1 mice. Diabetes. 1988 Feb;37(2):252–255. doi: 10.2337/diab.37.2.252. [DOI] [PubMed] [Google Scholar]
- Shah S. C., Malone J. I., Simpson N. E. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989 Mar 2;320(9):550–554. doi: 10.1056/NEJM198903023200902. [DOI] [PubMed] [Google Scholar]
- Stiller C. R., Dupré J., Gent M., Jenner M. R., Keown P. A., Laupacis A., Martell R., Rodger N. W., von Graffenried B., Wolfe B. M. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science. 1984 Mar 30;223(4643):1362–1367. doi: 10.1126/science.6367043. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]



