Abstract
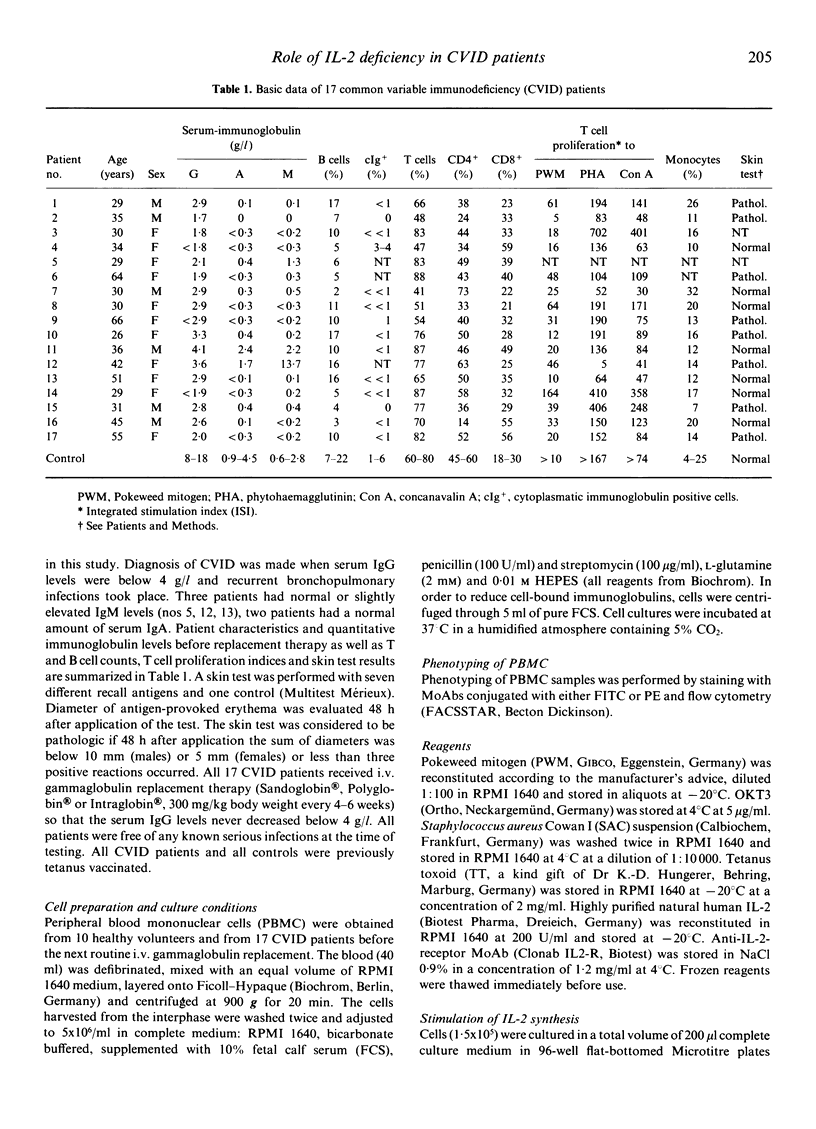
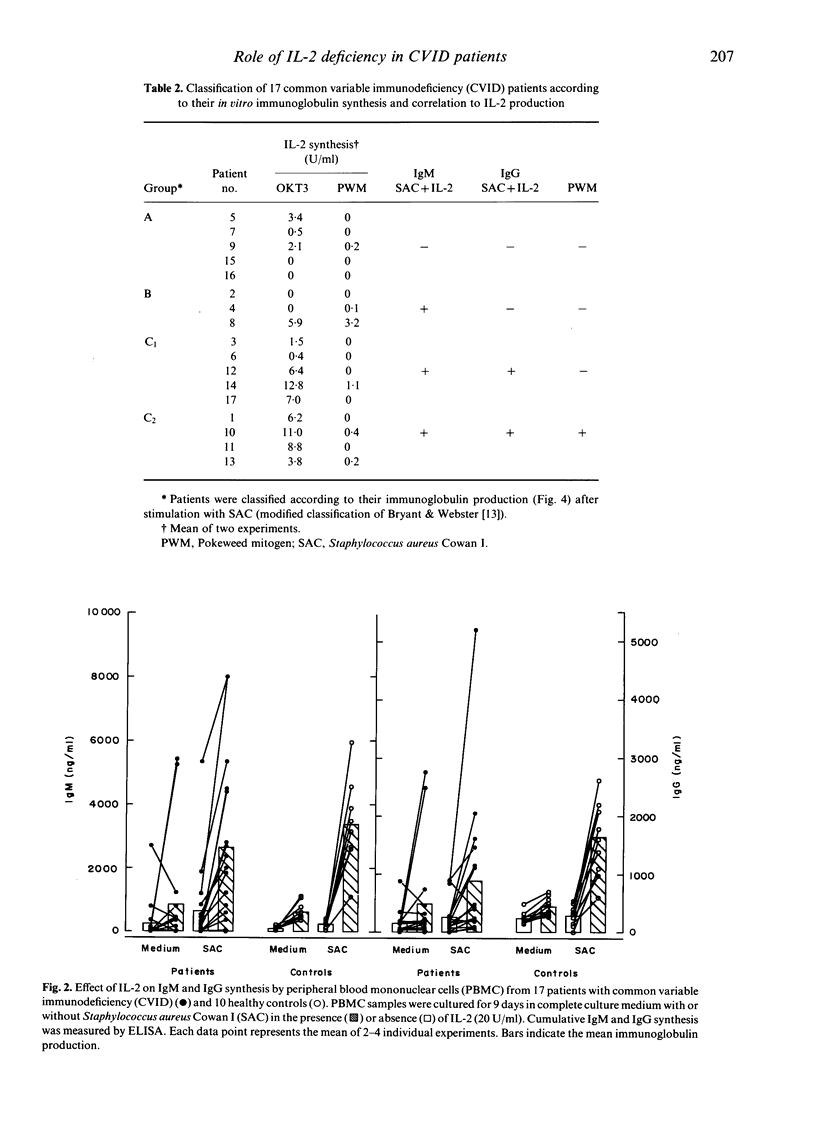
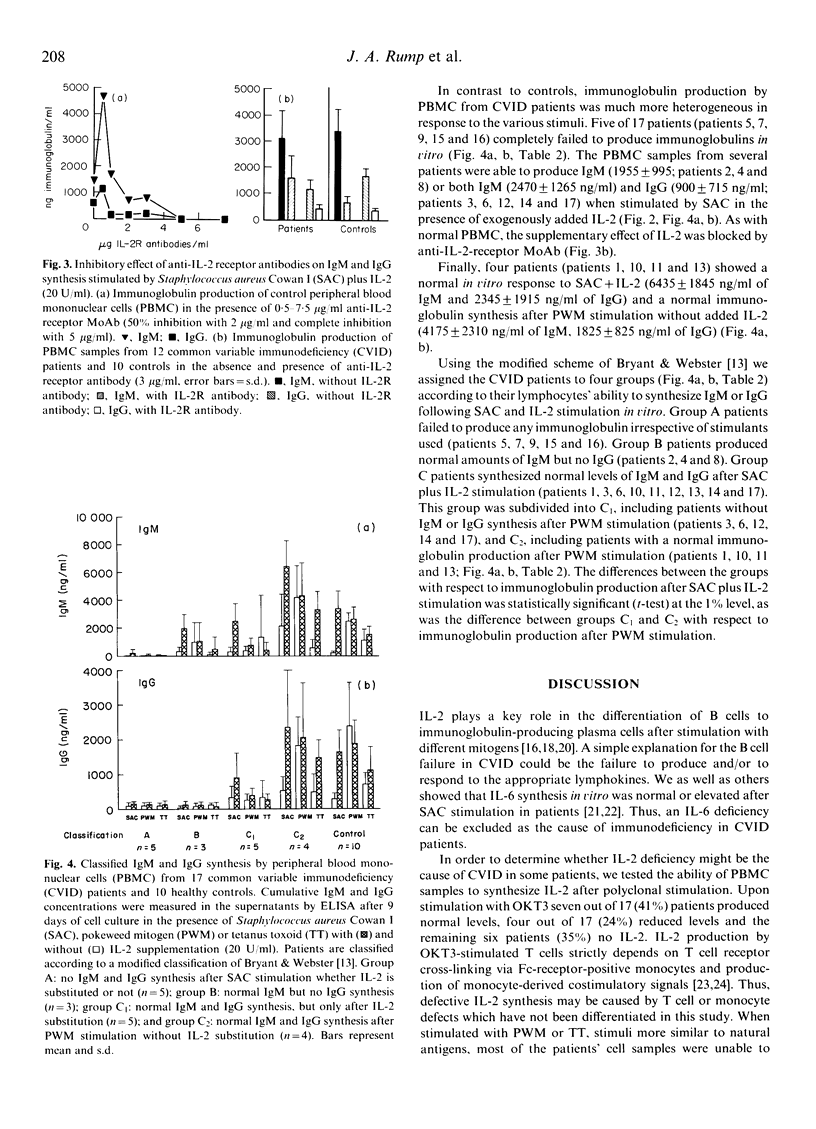
Common variable immunodeficiency (CVID) patients are unable to produce specific immunoglobulins after antigen contact in vivo. The aim of this study was to investigate whether in some cases of CVID a decreased de novo synthesis of IL-2 might be the cause of immunodeficiency and whether this deficiency can be corrected by IL-2 supplementation in vitro. Mononuclear cells from 17 CVID patients and from 10 healthy controls were cultured with monoclonal anti-CD3 antibody OKT3, pokeweed mitogen (PWM) or tetanus toxoid (TT) to stimulate IL-2 synthesis. In parallel, in vitro IgG and IgM synthesis was stimulated with Staphylococcus aureus Cowan I (SAC), PWM or TT in the presence or absence of IL-2. While lymphocytes of 11 out of 17 patients produced low to normal amounts of IL-2 upon stimulation with anti-CD3, only three patients showed low IL-2 production in response to PWM and five in response to TT. Regarding immunoglobulin synthesis in vitro, five patients completely failed to produce IgM or IgG upon stimulation with PWM, SAC or TT irrespective of the addition of IL-2. By contrast, four patients did not show any defect in vitro and synthesized normal amounts of IgM and IgG with any of the three stimuli. Finally, eight patients could be reconstituted for PWM-, SAC- and TT-induced IgM and/or IgG synthesis in vitro, by adding IL-2 to the culture system. This enhancing effect of IL-2 could be blocked by adding anti-IL-2 receptor antibodies to the cultures. Our findings indicate that a defective IL-2 synthesis after antigen stimulation may be one reason for the impaired immunoglobulin production in some cases of CVID.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman D. C., Matsuda T., Hirano T., Kishimoto T., Saxon A. Elevated serum interleukin-6 associated with a failure in B cell differentiation in common variable immunodeficiency. J Allergy Clin Immunol. 1990 Oct;86(4 Pt 1):512–521. doi: 10.1016/s0091-6749(05)80207-6. [DOI] [PubMed] [Google Scholar]
- Ariga T., Okano M., Takahashi Y., Sakiyama Y., Matsumoto S. Analysis of B cell dysfunction in patients with common variable immunodeficiency by using recombinant interleukin 2. Tohoku J Exp Med. 1987 May;152(1):53–61. doi: 10.1620/tjem.152.53. [DOI] [PubMed] [Google Scholar]
- Bryant A., Calver N. C., Toubi E., Webster A. D., Farrant J. Classification of patients with common variable immunodeficiency by B cell secretion of IgM and IgG in response to anti-IgM and interleukin-2. Clin Immunol Immunopathol. 1990 Aug;56(2):239–248. doi: 10.1016/0090-1229(90)90145-g. [DOI] [PubMed] [Google Scholar]
- Ceuppens J. L., Bloemmen F. J., Van Wauwe J. P. T cell unresponsiveness to the mitogenic activity of OKT3 antibody results from a deficiency of monocyte Fc gamma receptors for murine IgG2a and inability to cross-link the T3-Ti complex. J Immunol. 1985 Dec;135(6):3882–3886. [PubMed] [Google Scholar]
- De Gast G. C., Wilkins S. R., Webster A. D., Rickinson A., Platts-Mills T. A. Functional 'immaturity' of isolated B cells from patients with hypogammaglobulinaemia. Clin Exp Immunol. 1980 Dec;42(3):535–544. [PMC free article] [PubMed] [Google Scholar]
- Eibl M. M., Mannhalter J. W., Zlabinger G., Mayr W. R., Tilz G. P., Ahmad R., Zielinski C. C. Defective macrophage function in a patient with common variable immunodeficiency. N Engl J Med. 1982 Sep 23;307(13):803–806. doi: 10.1056/NEJM198209233071307. [DOI] [PubMed] [Google Scholar]
- Farrant J., Bryant A. E., Lever A. M., Edwards A. J., Knight S. C., Webster A. D. Defective low-density cells of dendritic morphology from the blood of patients with common variable hypogammaglobulinaemia: low immunoglobulin production on stimulation of normal B cells. Clin Exp Immunol. 1985 Jul;61(1):189–194. [PMC free article] [PubMed] [Google Scholar]
- Farrant J., Bryant A., Almandoz F., Spickett G., Evans S. W., Webster A. D. B cell function in acquired "common-variable" hypogammaglobulinemia: proliferative responses to lymphokines. Clin Immunol Immunopathol. 1989 May;51(2):196–204. doi: 10.1016/0090-1229(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Gerrard T. L., Fauci A. S. Activation and immunoregulation of antigen-specific human b lymphocyte responses: multifaceted role of the monocyte. J Immunol. 1982 May;128(5):2367–2372. [PubMed] [Google Scholar]
- Lebranchu Y., Thibault G., Degenne D., Bardos P. Abnormalities in CD4+ T lymphocyte subsets in patients with common variable immunodeficiency. Clin Immunol Immunopathol. 1991 Oct;61(1):83–92. doi: 10.1016/s0090-1229(06)80009-7. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Meyer zum Büschenfelde K. H. T cell receptor triggering induces responsiveness to interleukin 1 and interleukin 2 but does not lead to T cell proliferation. J Immunol. 1986 Jun 1;136(11):4106–4112. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- North M. E., Spickett G. P., Allsop J., Webster A. D., Farrant J. Defective DNA synthesis by T cells in acquired 'common-variable' hypogammaglobulinaemia on stimulation with mitogens. Clin Exp Immunol. 1989 Apr;76(1):19–23. [PMC free article] [PubMed] [Google Scholar]
- Pastorelli G., Roncarolo M. G., Touraine J. L., Peronne G., Tovo P. A., de Vries J. E. Peripheral blood lymphocytes of patients with common variable immunodeficiency (CVI) produce reduced levels of interleukin-4, interleukin-2 and interferon-gamma, but proliferate normally upon activation by mitogens. Clin Exp Immunol. 1989 Dec;78(3):334–340. [PMC free article] [PubMed] [Google Scholar]
- Rump J. A., Schlesier M., Wolf-Vorbeck G., Dräger R., Melchers I., Peter H. H. Effekte von IL-2 und IL-6 auf die Immunglobulin-synthese von Lymphozyten von CVID-Patienten. Immun Infekt. 1990 Jun;18(3):93–95. [PubMed] [Google Scholar]
- Röther E., Vaith P., Peter H. H. Isolierter Immunglobulinmangel G (IgG) bei Dystrophia myotonica (Curschmann-Steinert). Med Klin (Munich) 1990 Mar;85 (Suppl 1):150–153. [PubMed] [Google Scholar]
- Saiki O., Ralph P., Cunningham-Rundles C., Good R. A. Three distinct stages of B-cell defects in common varied immunodeficiency. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6008–6012. doi: 10.1073/pnas.79.19.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerwein R. W., Mudde G. C., Vooys W. C., van der Meer W. G., de Gast G. C., Aarden L. A. Induction of IgM production by pokeweed mitogen and interleukin-2: different responses by human peripheral blood cells and tonsil cells. Clin Immunol Immunopathol. 1986 Jun;39(3):431–441. doi: 10.1016/0090-1229(86)90171-6. [DOI] [PubMed] [Google Scholar]
- Spickett G. P., Webster A. D., Farrant J. Cellular abnormalities in common variable immunodeficiency. Immunodefic Rev. 1990;2(3):199–219. [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Teranishi T., Hirano T., Lin B. H., Onoue K. Demonstration of the involvement of interleukin 2 in the differentiation of Staphylococcus aureus Cowan I-stimulated B cells. J Immunol. 1984 Dec;133(6):3062–3067. [PubMed] [Google Scholar]
- Waldmann T. A., Broder S., Krakauer R., MacDermott R. P., Durm M., Goldman C., Meade B. The role of suppressor cells in the pathogenesis of common variable hypogammaglobulinemia and the immunodeficiency associated with myeloma. Fed Proc. 1976 Jul;35(9):2067–2072. [PubMed] [Google Scholar]
- Wochner R. D., Drews G., Strober W., Waldmann T. A. Accelerated breakdown of immunoglobulin G (IgG) in myotonic dystrophy: a hereditary error of immunoglobulin catabolism. J Clin Invest. 1966 Mar;45(3):321–329. doi: 10.1172/JCI105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Concha E. G., Oldham G., Webster A. D., Asherson G. L., Platts-Mills T. A. Quantitative measurements of T- and B-cell function in "variable" primary hypogammaglobulinaemia: evidence for a consistent B-cell defect. Clin Exp Immunol. 1977 Feb;27(2):208–215. [PMC free article] [PubMed] [Google Scholar]
- von Bismarck U., Peest D., Dräger R., Serbin A., Schlesier M., Peter H. H. Terminale B-Zellreifung und Immunoglobulinsynthese in vitro bei primären und sekundären Immundefekten. Immun Infekt. 1984 Apr;12(2):75–87. [PubMed] [Google Scholar]



