Abstract
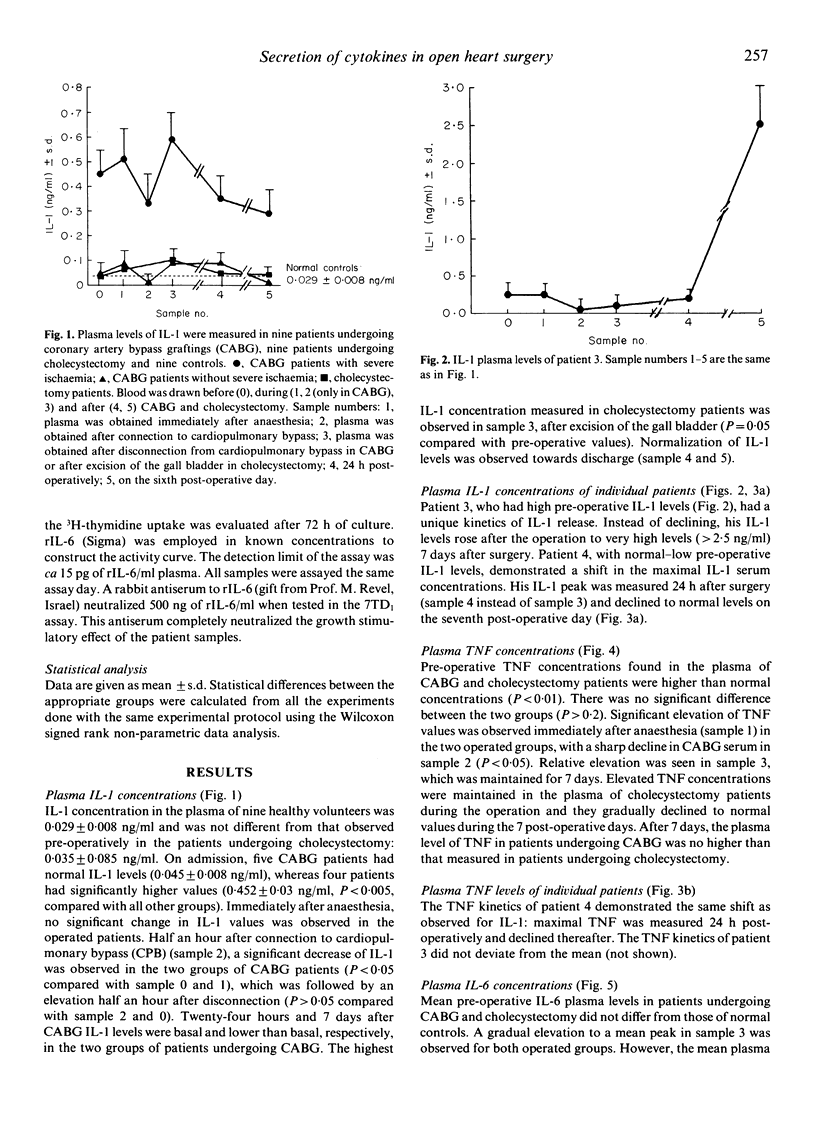
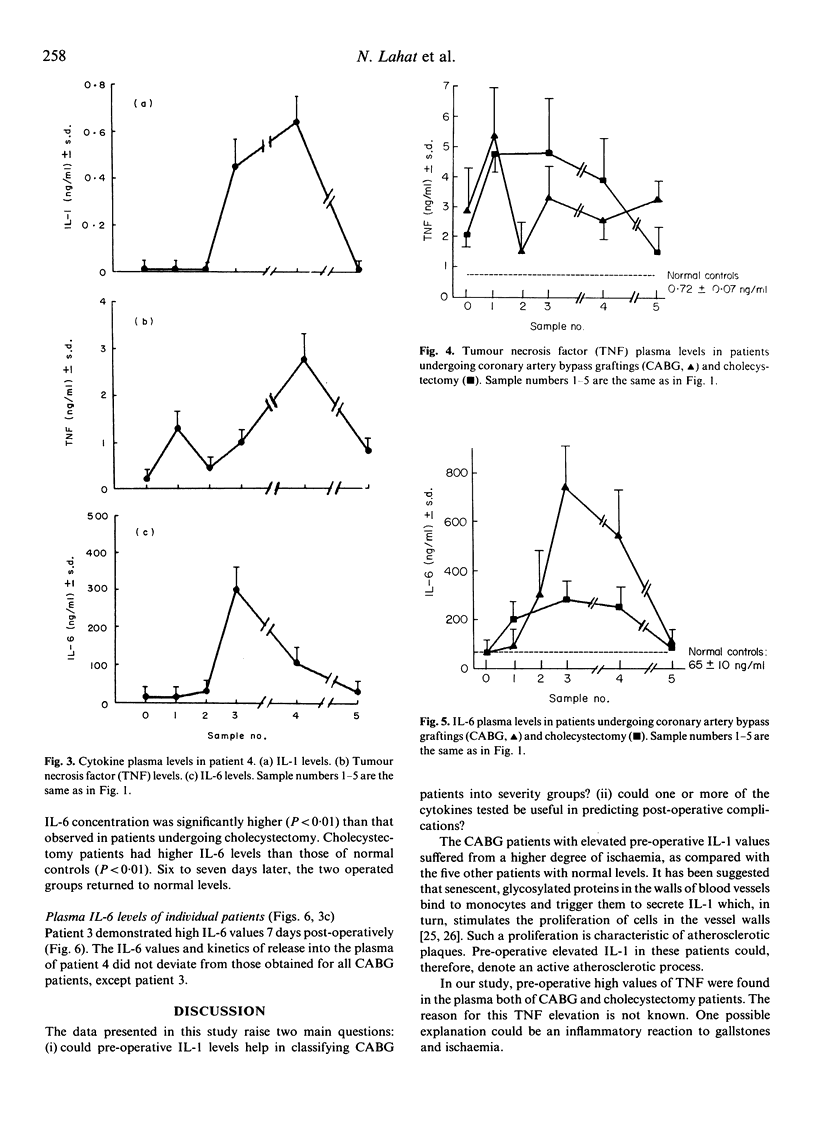
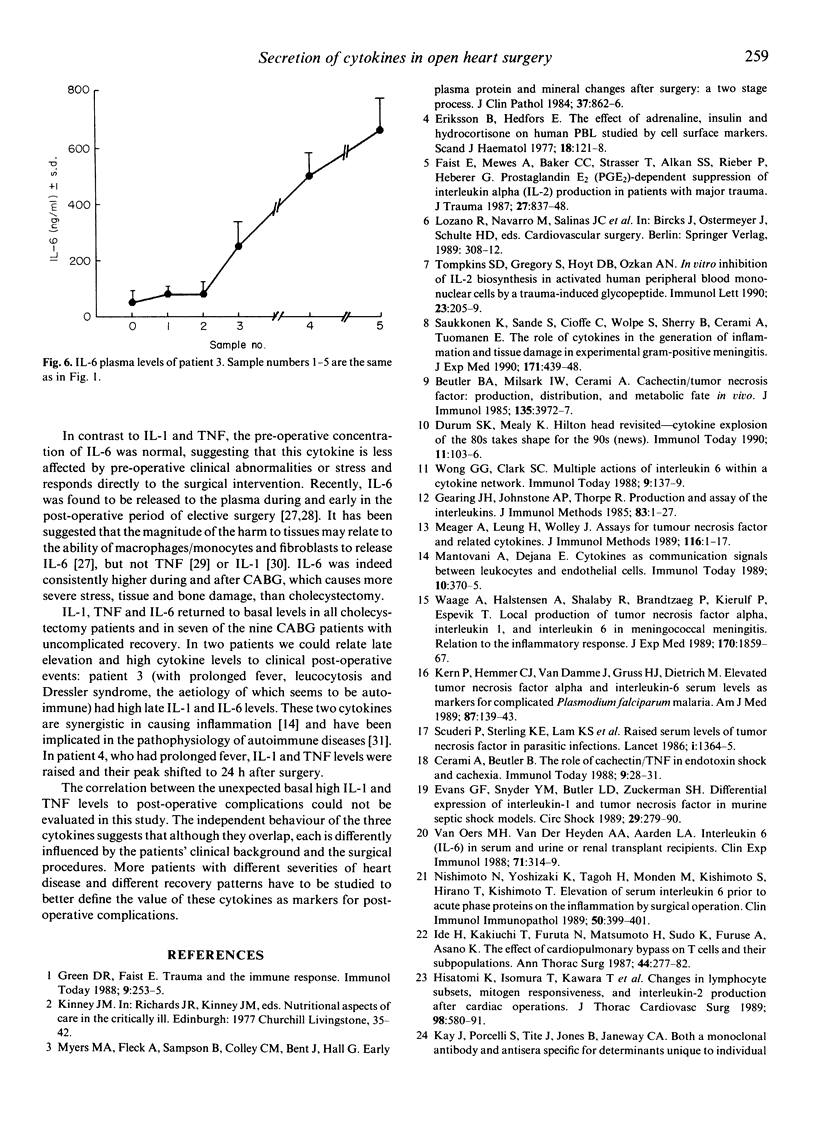
Plasma levels of biologically active IL-1, tumour necrosis factor (TNF) and IL-6 were measured before, during and after coronary artery bypass graftings (CABG) (n = 9) and cholecystectomy (CHO, n = 9), and in normal controls (nine healthy volunteers). Mean pre-operative IL-1 concentration in four of the nine CABG patients was 0.452 + 0.03 ng/ml, significantly (P less than 0.001) higher than that of the other five (0.045 +/- 0.009 ng/ml), CHO patients (0.035 +/- 0.005 ng/ml) and controls (0.029 +/- 0.008 ng/ml). Three of the four patients with high pre-operative IL-1 had functional capacity IV, while the other five had functional capacity IIa or IIb. Slight IL-1 elevation after anaesthesia, followed by reduction after initiation of bypass, elevation on completion of surgery and reduction to basal levels after 7 days was found in patients undergoing CABG. Mean basal TNF levels of CABG and CHO patients did not differ, but were higher than those of controls (2.85 +/- 0.5 ng/ml for CABG, 2.05 +/- 0.06 ng/ml for CHO, 0.72 +/- 0.07 ng/ml for normals, P less than 0.001). A unique kinetics of release during CABG was observed also for TNF. Mean pre-operative IL-6 levels were normal (50 +/- 3 ng/ml for CABG, 50 +/- 0.5 ng/ml for CHO and 65 +/- 10 ng/ml for controls). Gradual elevation to a mean peak of 725 +/- 100 ng/ml on completion of CABG was observed as compared with 275 +/- 50 ng/ml in CHO (P less than 0.01). On the seventh post-operative day mean IL-6 levels returned to normal. Two patients with post-operative low-grade fever (38 degrees C) had high, late cytokine levels. One of these two patients had leucocytosis, sterile discharge from the operative wound and was diagnosed as suffering from the Dressler syndrome. In this study elevated cytokine values and unique kinetics of release into the serum were found in patients undergoing CABG.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Bitterman H., Kinarty A., Lazarovich H., Lahat N. Acute release of cytokines is proportional to tissue injury induced by surgical trauma and shock in rats. J Clin Immunol. 1991 Jul;11(4):184–192. doi: 10.1007/BF00917424. [DOI] [PubMed] [Google Scholar]
- Cerami A., Beutler B. The role of cachectin/TNF in endotoxic shock and cachexia. Immunol Today. 1988 Jan;9(1):28–31. doi: 10.1016/0167-5699(88)91353-9. [DOI] [PubMed] [Google Scholar]
- Durum S. K., Mealy K. Hilton Head revisited--cytokine explosion of the 80s takes shape for the 90s. Immunol Today. 1990 Apr;11(4):103–106. doi: 10.1016/0167-5699(90)90037-a. [DOI] [PubMed] [Google Scholar]
- Eriksson B., Hedfors E. The effect of adrenaline, insulin and hydrocortisone on human peripheral blood lymphocytes studied by cell surface markers. Scand J Haematol. 1977 Feb;18(2):121–128. doi: 10.1111/j.1600-0609.1977.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Evans G. F., Snyder Y. M., Butler L. D., Zuckerman S. H. Differential expression of interleukin-1 and tumor necrosis factor in murine septic shock models. Circ Shock. 1989 Dec;29(4):279–290. [PubMed] [Google Scholar]
- Faist E., Mewes A., Baker C. C., Strasser T., Alkan S. S., Rieber P., Heberer G. Prostaglandin E2 (PGE2)-dependent suppression of interleukin alpha (IL-2) production in patients with major trauma. J Trauma. 1987 Aug;27(8):837–848. doi: 10.1097/00005373-198708000-00001. [DOI] [PubMed] [Google Scholar]
- Fuster V., Badimon L., Badimon J. J., Chesebro J. H. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992 Jan 23;326(4):242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Johnstone A. P., Thorpe R. Production and assay of the interleukins. J Immunol Methods. 1985 Oct 24;83(1):1–27. doi: 10.1016/0022-1759(85)90053-5. [DOI] [PubMed] [Google Scholar]
- Green D. R., Faist E. Trauma and the immune response. Immunol Today. 1988 Sep;9(9):253–255. doi: 10.1016/0167-5699(88)91300-X. [DOI] [PubMed] [Google Scholar]
- Hack C. E., De Groot E. R., Felt-Bersma R. J., Nuijens J. H., Strack Van Schijndel R. J., Eerenberg-Belmer A. J., Thijs L. G., Aarden L. A. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989 Oct;74(5):1704–1710. [PubMed] [Google Scholar]
- Hisatomi K., Isomura T., Kawara T., Yamashita M., Hirano A., Yoshida H., Eriguchi N., Kosuga K., Ohishi K. Changes in lymphocyte subsets, mitogen responsiveness, and interleukin-2 production after cardiac operations. J Thorac Cardiovasc Surg. 1989 Oct;98(4):580–591. [PubMed] [Google Scholar]
- Ide H., Kakiuchi T., Furuta N., Matsumoto H., Sudo K., Furuse A., Asano K. The effect of cardiopulmonary bypass on T cells and their subpopulations. Ann Thorac Surg. 1987 Sep;44(3):277–282. doi: 10.1016/s0003-4975(10)62074-7. [DOI] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P., Hemmer C. J., Van Damme J., Gruss H. J., Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989 Aug;87(2):139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. B-cell stimulatory factors (BSFs): molecular structure, biological function, and regulation of expression. J Clin Immunol. 1987 Sep;7(5):343–355. doi: 10.1007/BF00917012. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989 Nov;10(11):370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Cytokines are two-edged swords in disease. Science. 1988 Jan 15;239(4837):257–258. doi: 10.1126/science.2827307. [DOI] [PubMed] [Google Scholar]
- Meager A., Leung H., Woolley J. Assays for tumour necrosis factor and related cytokines. J Immunol Methods. 1989 Jan 6;116(1):1–17. doi: 10.1016/0022-1759(89)90306-2. [DOI] [PubMed] [Google Scholar]
- Myers M. A., Fleck A., Sampson B., Colley C. M., Bent J., Hall G. Early plasma protein and mineral changes after surgery: a two stage process. J Clin Pathol. 1984 Aug;37(8):862–866. doi: 10.1136/jcp.37.8.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N., Yoshizaki K., Tagoh H., Monden M., Kishimoto S., Hirano T., Kishimoto T. Elevation of serum interleukin 6 prior to acute phase proteins on the inflammation by surgical operation. Clin Immunol Immunopathol. 1989 Mar;50(3):399–401. doi: 10.1016/0090-1229(89)90147-5. [DOI] [PubMed] [Google Scholar]
- Pullicino E. A., Carli F., Poole S., Rafferty B., Malik S. T., Elia M. The relationship between the circulating concentrations of interleukin 6 (IL-6), tumor necrosis factor (TNF) and the acute phase response to elective surgery and accidental injury. Lymphokine Res. 1990 Summer;9(2):231–238. [PubMed] [Google Scholar]
- Saukkonen K., Sande S., Cioffe C., Wolpe S., Sherry B., Cerami A., Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990 Feb 1;171(2):439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi P., Sterling K. E., Lam K. S., Finley P. R., Ryan K. J., Ray C. G., Petersen E., Slymen D. J., Salmon S. E. Raised serum levels of tumour necrosis factor in parasitic infections. Lancet. 1986 Dec 13;2(8520):1364–1365. doi: 10.1016/s0140-6736(86)92007-6. [DOI] [PubMed] [Google Scholar]
- Shenkin A., Fraser W. D., Series J., Winstanley F. P., McCartney A. C., Burns H. J., Van Damme J. The serum interleukin 6 response to elective surgery. Lymphokine Res. 1989 Summer;8(2):123–127. [PubMed] [Google Scholar]
- Tompkins S. D., Gregory S., Hoyt D. B., Ozkan A. N. In vitro inhibition of IL-2 biosynthesis in activated human peripheral blood mononuclear cells by a trauma-induced glycopeptide. Immunol Lett. 1990 Jan;23(3):205–209. doi: 10.1016/0165-2478(90)90193-t. [DOI] [PubMed] [Google Scholar]
- Van Oers M. H., Van der Heyden A. A., Aarden L. A. Interleukin 6 (IL-6) in serum and urine of renal transplant recipients. Clin Exp Immunol. 1988 Feb;71(2):314–319. [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Shalaby R., Brandtzaeg P., Kierulf P., Espevik T. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989 Dec 1;170(6):1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]



