Abstract
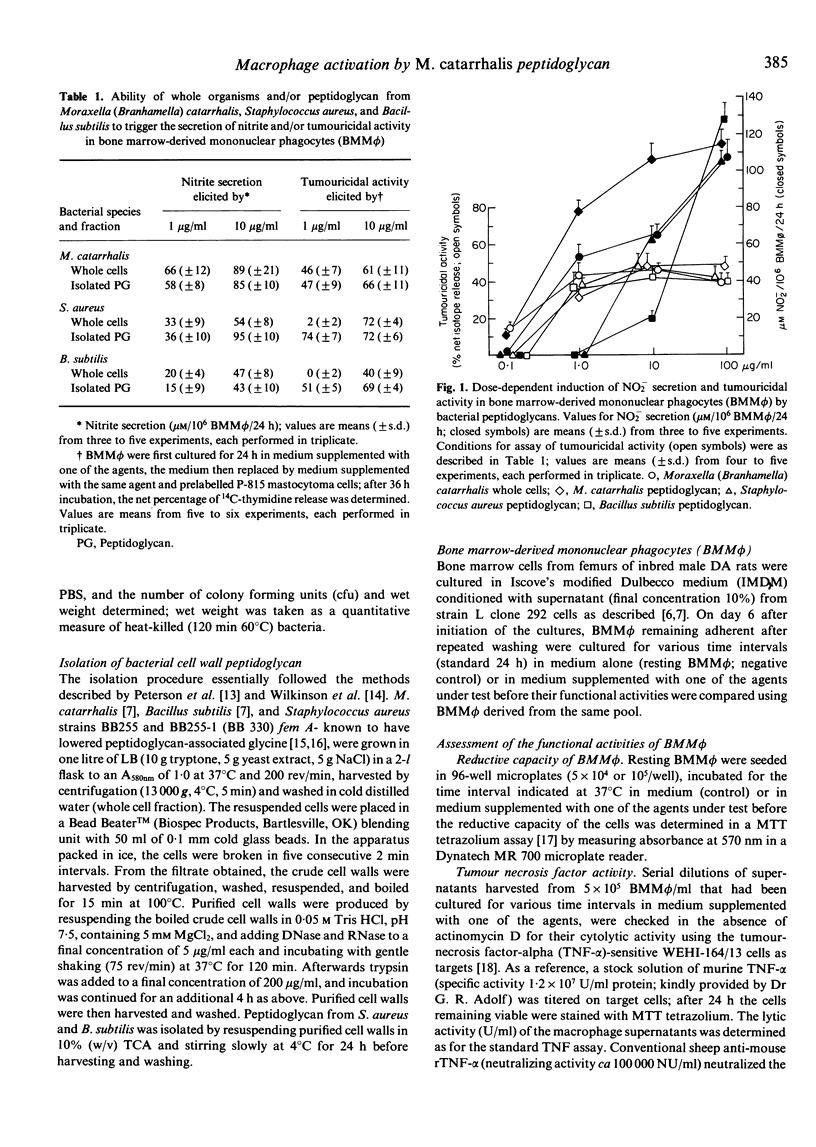
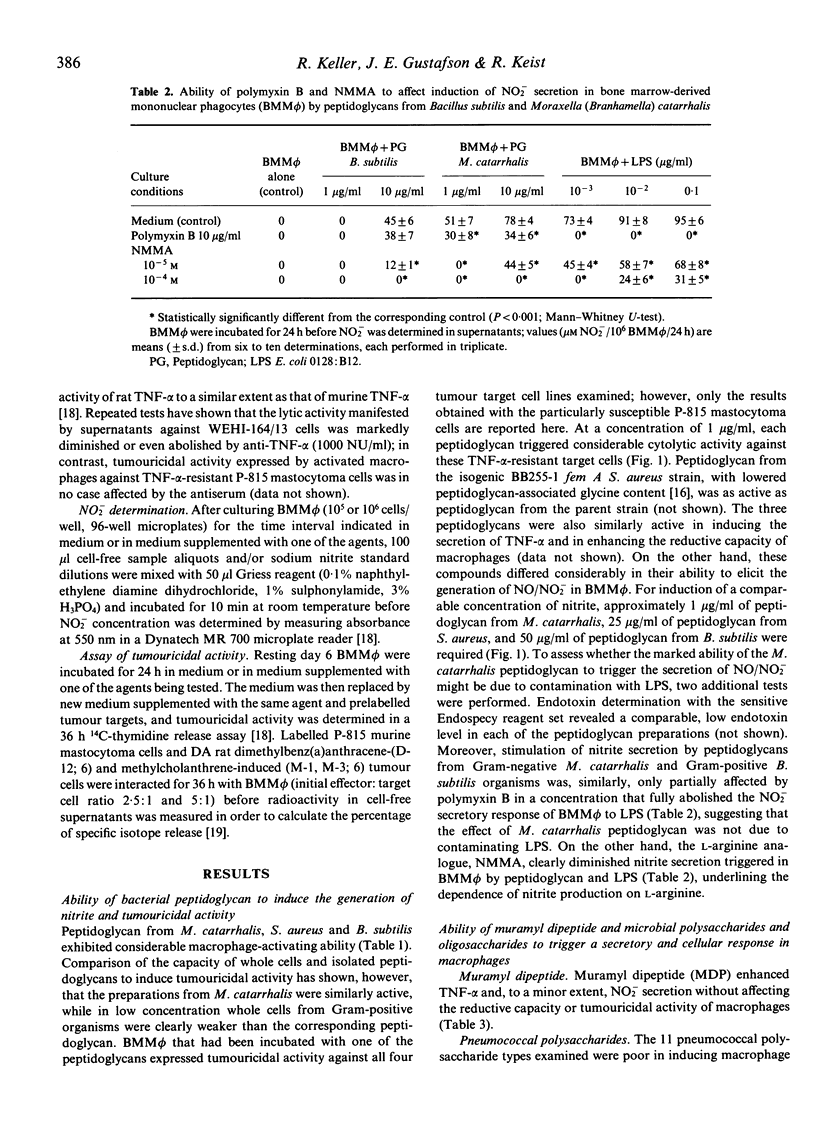
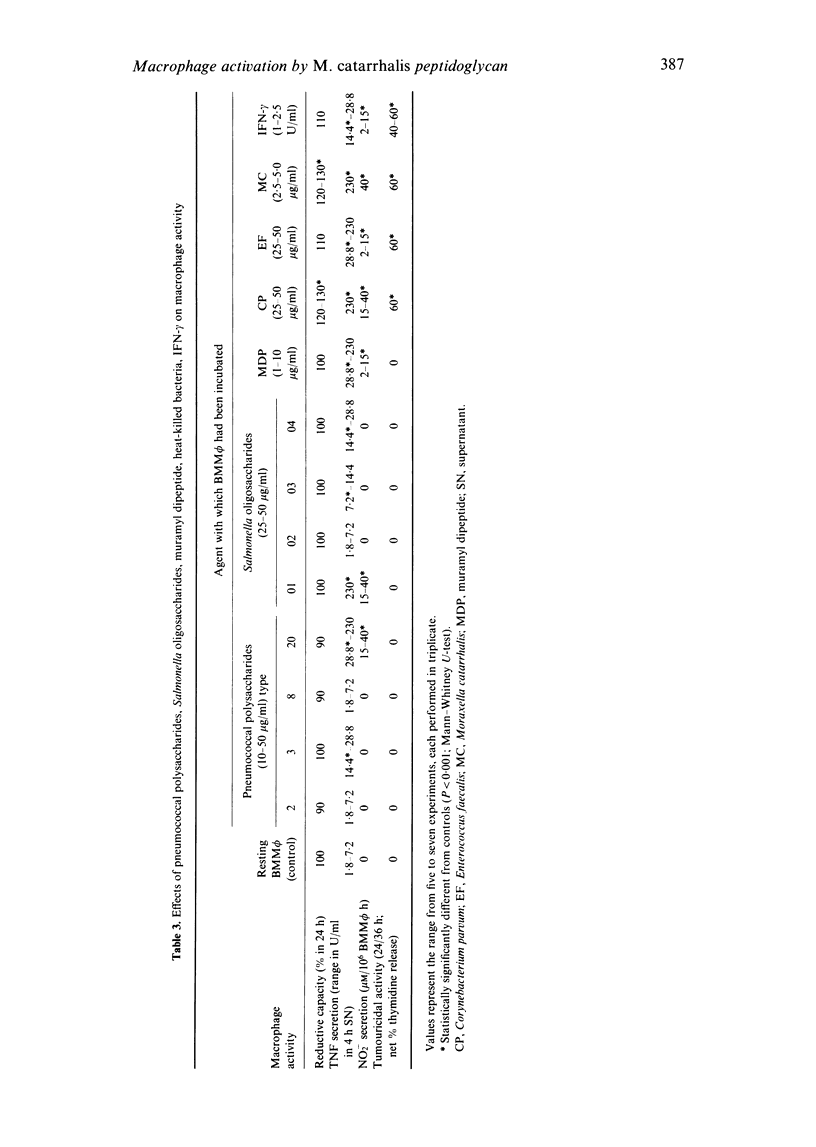
Moraxella (Branhamella) catarrhalis organisms have been shown to be particularly efficient in inducing in a pure population of bone marrow-derived mononuclear phagocytes secretory and cellular activities. In the present study, the ability of peptidoglycan from this Gram-negative organism to trigger a macrophage response was compared with that elicited by peptidoglycan from Staphylococcus aureus and Bacillus subtilis. The results show that the three peptidoglycans were similarly active in triggering the secretion of tumour necrosis factor and tumouricidal activity but differed considerably in their ability to induce the generation of nitrite in macrophages; in this respect, peptidoglycan from M. catarrhalis was particularly potent. The impressive capacity of M. catarrhalis peptidoglycan to induce in low concentration the secretion of tumour necrosis factor and nitrite and tumouricidal activity may, in addition to its lipopolysaccharide, contribute to the extraordinary potential of this organism to trigger the functional activities of macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Ciorbaru R., Ellouz F., Petit J. F., Lederer E. Adjuvant activity of monomeric bacterial cell wall peptidoglycans. Biochem Biophys Res Commun. 1974 Feb 4;56(3):561–567. doi: 10.1016/0006-291x(74)90640-8. [DOI] [PubMed] [Google Scholar]
- Berger-Bächi B., Strässle A., Kayser F. H. Characterization of an isogenic set of methicillin-resistant and susceptible mutants of Staphylococcus aureus. Eur J Clin Microbiol. 1986 Dec;5(6):697–701. doi: 10.1007/BF02013308. [DOI] [PubMed] [Google Scholar]
- Bock K., Meldal M., Bundle D. R., Iversen T., Pinto B. M., Garegg P. J., Kvanström I., Norberg T., Lindberg A. A., Svenson S. B. The conformation of Salmonella O-antigenic oligosaccharides of serogroups A, B, and D1 inferred from 1H- and 13C-nuclear magnetic resonance spectroscopy. Carbohydr Res. 1984 Jul 15;130:35–53. doi: 10.1016/0008-6215(84)85268-4. [DOI] [PubMed] [Google Scholar]
- Byars N. E. Two adjuvant-active muramyl dipeptide analogs induce differential production of lymphocyte-activating factor and a factor causing distress in guinea pigs. Infect Immun. 1984 May;44(2):344–350. doi: 10.1128/iai.44.2.344-350.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Glauner B. Structure and metabolism of the murein sacculus. Res Microbiol. 1990 Jan;141(1):75–89. doi: 10.1016/0923-2508(90)90100-5. [DOI] [PubMed] [Google Scholar]
- Jansson P. E., Lindberg B., Anderson M., Lindquist U., Henrichsen J. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 2, a reinvestigation. Carbohydr Res. 1988 Oct 15;182(1):111–117. doi: 10.1016/0008-6215(88)84095-3. [DOI] [PubMed] [Google Scholar]
- Keller R., Gehri R., Keist R., Huf E., Kayser F. H. The interaction of macrophages and bacteria: a comparative study of the induction of tumoricidal activity and of reactive nitrogen intermediates. Cell Immunol. 1991 Apr 15;134(1):249–256. doi: 10.1016/0008-8749(91)90348-f. [DOI] [PubMed] [Google Scholar]
- Keller R., Gehri R., Keist R. The interaction of macrophages and bacteria: Escherichia coli species, bacterial lipopolysaccharide, and lipid A differ in their ability to induce tumoricidal activity and the secretion of reactive nitrogen intermediates in macrophages. Cell Immunol. 1992 Apr 15;141(1):47–58. doi: 10.1016/0008-8749(92)90126-a. [DOI] [PubMed] [Google Scholar]
- Keller R., Geiges M., Keist R. L-arginine-dependent reactive nitrogen intermediates as mediators of tumor cell killing by activated macrophages. Cancer Res. 1990 Mar 1;50(5):1421–1425. [PubMed] [Google Scholar]
- Keller R., Keist R., Frei K. Lymphokines and bacteria, that induce tumoricidal activity, trigger a different secretory response in macrophages. Eur J Immunol. 1990 Mar;20(3):695–698. doi: 10.1002/eji.1830200334. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Klauser S., Schweiger A. The macrophage response to bacteria: flow of L-arginine through the nitric oxide and urea pathways and induction of tumoricidal activity. Biochem Biophys Res Commun. 1991 Jun 14;177(2):821–827. doi: 10.1016/0006-291x(91)91863-8. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Van der Meide P. H., Groscurth P., Aguet M., Leist T. P. Induction, maintenance, and reinduction of tumoricidal activity in bone marrow-derived mononuclear phagocytes by Corynebacterium parvum. Evidence for the involvement of a T cell- and interferon-gamma-independent pathway of macrophage activation. J Immunol. 1987 Apr 1;138(7):2366–2371. [PubMed] [Google Scholar]
- Keller R., Keist R., Wechsler A., Leist T. P., van der Meide P. H. Mechanisms of macrophage-mediated tumor cell killing: a comparative analysis of the roles of reactive nitrogen intermediates and tumor necrosis factor. Int J Cancer. 1990 Oct 15;46(4):682–686. doi: 10.1002/ijc.2910460422. [DOI] [PubMed] [Google Scholar]
- Labischinski H., Goodell E. W., Goodell A., Hochberg M. L. Direct proof of a "more-than-single-layered" peptidoglycan architecture of Escherichia coli W7: a neutron small-angle scattering study. J Bacteriol. 1991 Jan;173(2):751–756. doi: 10.1128/jb.173.2.751-756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidhof H., Reinicke B., Blümel P., Berger-Bächi B., Labischinski H. femA, which encodes a factor essential for expression of methicillin resistance, affects glycine content of peptidoglycan in methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J Bacteriol. 1991 Jun;173(11):3507–3513. doi: 10.1128/jb.173.11.3507-3513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbaki N., Rikitomi N., Nagatake T., Matsumoto K. Correlation between Branhamella catarrhalis adherence to oropharyngeal cells and seasonal incidence of lower respiratory tract infections. Tohoku J Exp Med. 1987 Oct;153(2):111–121. doi: 10.1620/tjem.153.111. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nagatake T. Clinical significance of respiratory infection caused by Branhamella catarrhalis with special reference to beta-lactamase producing strains. Tohoku J Exp Med. 1985 Sep;147(1):1–13. doi: 10.1620/tjem.147.1. [DOI] [PubMed] [Google Scholar]
- Nauciel C., Fleck J., Martin J. P., Mock M., Nguyen-Huy H. Adjuvant activity of bacterial peptidoglycans on the production of delayed hypersensitivity and on antibody response. Eur J Immunol. 1974 May;4(5):352–356. doi: 10.1002/eji.1830040509. [DOI] [PubMed] [Google Scholar]
- Nauciel C., Goguel A. F. Inhibition of tumor growth by the peptidoglycan from Bacillus megaterium. J Natl Cancer Inst. 1977 Dec;59(6):1723–1726. doi: 10.1093/jnci/59.6.1723. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Douglas S. D., Quie P. G., Verhoef J. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J Clin Invest. 1978 Mar;61(3):597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. M., Lockwood B. M. A 2-year survey of Branhamella catarrhalis in a general hospital. J Hosp Infect. 1986 May;7(3):277–282. doi: 10.1016/0195-6701(86)90078-2. [DOI] [PubMed] [Google Scholar]
- Stewart-Tull D. E. The immunological activities of bacterial peptidoglycans. Annu Rev Microbiol. 1980;34:311–340. doi: 10.1146/annurev.mi.34.100180.001523. [DOI] [PubMed] [Google Scholar]
- Svenson S. B., Lönngren J., Carlin N., Lindberg A. A. Salmonella bacteriophage glycanases: endorhamnosidases of Salmonella typhimurium bacteriophages. J Virol. 1979 Nov;32(2):583–592. doi: 10.1128/jvi.32.2.583-592.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacheron F., Guenounou M., Nauciel C. Induction of interleukin 1 secretion by adjuvant-active peptidoglycans. Infect Immun. 1983 Dec;42(3):1049–1054. doi: 10.1128/iai.42.3.1049-1054.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hare G. F., Shurin P. A., Marchant C. D., Cartelli N. A., Johnson C. E., Fulton D., Carlin S., Kim C. H. Acute otitis media caused by Branhamella catarrhalis: biology and therapy. Rev Infect Dis. 1987 Jan-Feb;9(1):16–27. doi: 10.1093/clinids/9.1.16. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]
- Wallace R. J., Jr, Musher D. M. In honor of Dr. Sarah Branham, a star is born. The realization of Branhamella catarrhalis as a respiratory pathogen. Chest. 1986 Sep;90(3):447–450. doi: 10.1378/chest.90.3.447. [DOI] [PubMed] [Google Scholar]
- Wientjes F. B., Woldringh C. L., Nanninga N. Amount of peptidoglycan in cell walls of gram-negative bacteria. J Bacteriol. 1991 Dec;173(23):7684–7691. doi: 10.1128/jb.173.23.7684-7691.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K., Quie P. G., Michael A. F. Activation of complement by cell surface components of Staphylococcus aureus. Infect Immun. 1978 May;20(2):388–392. doi: 10.1128/iai.20.2.388-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


