Abstract
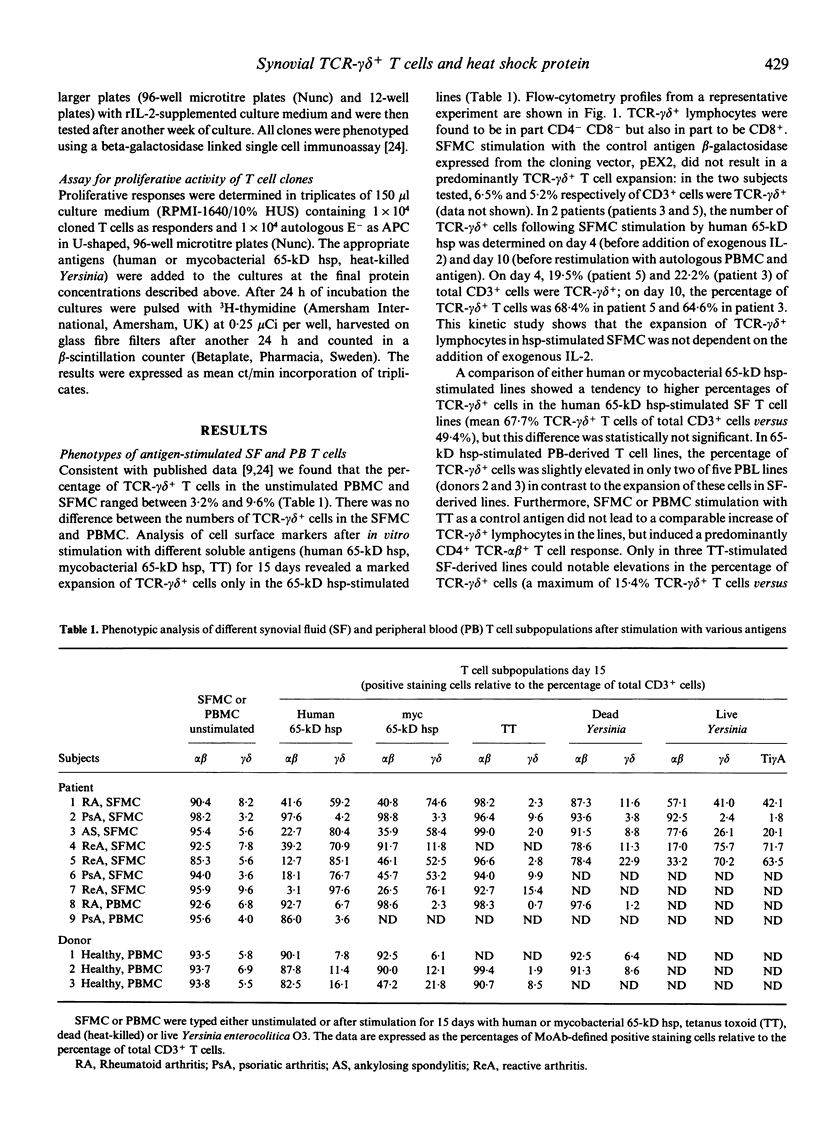
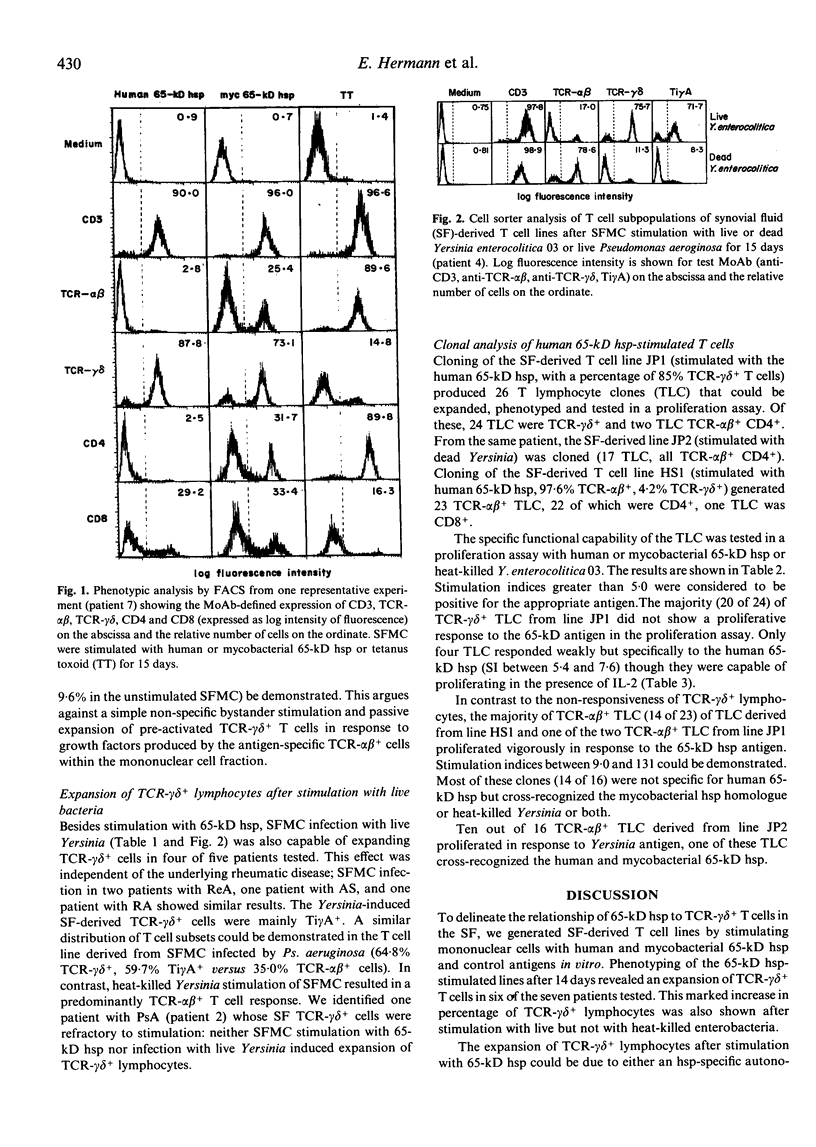
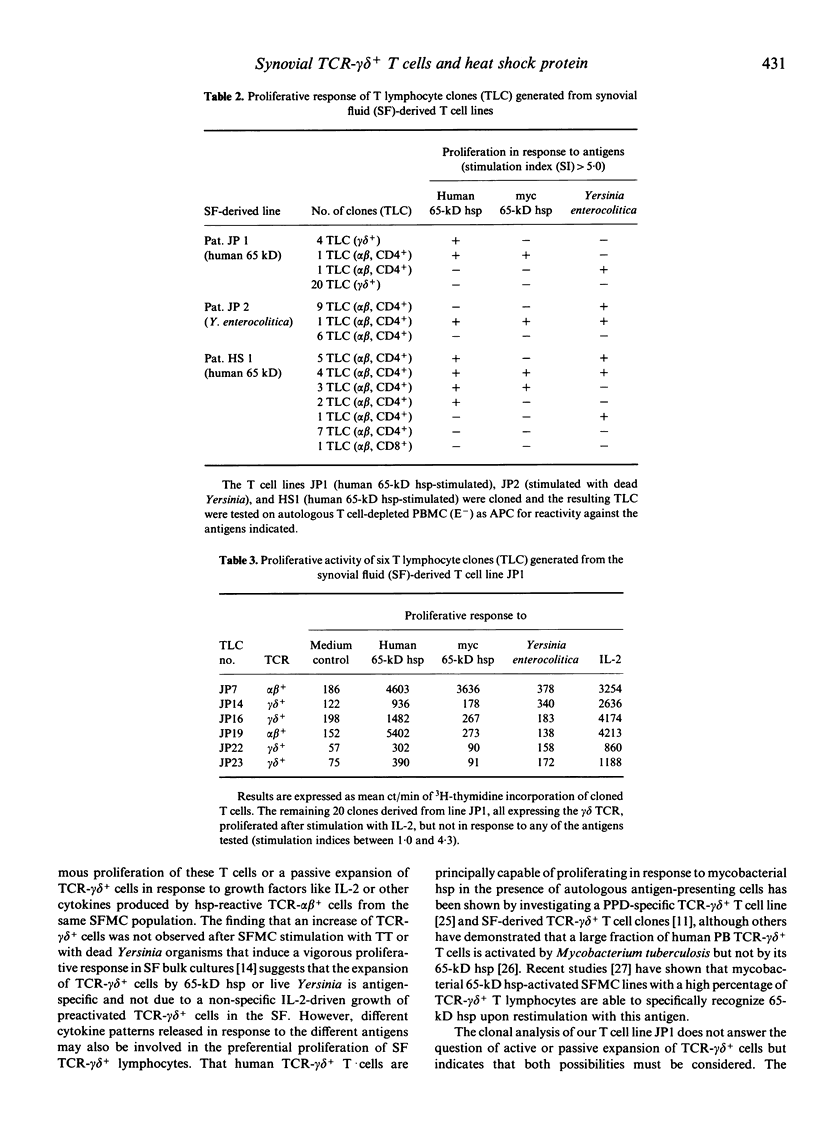
T lymphocyte responses to heterologous or self 65-kD heat shock protein (hsp) have been implicated in the pathogenesis of various forms of arthritis. To delineate the relationship of 65-kD hsp to different synovial fluid (SF) T cell subsets, we stimulated synovial fluid (SFMC) and peripheral blood mononuclear cells (PBMC) from patients with different inflammatory rheumatic diseases and from healthy controls with human or mycobacterial 65-kD hsp, tetanus toxoid (TT), heat-killed or live Yersinia enterocolitica. Phenotyping of the resulting T cell lines revealed an increase of up to 97% TCR-gamma delta+ lymphocytes in the 65-kD hsp-stimulated SF-derived lines. This expansion of TCR-gamma delta+ cells was less pronounced with cultures of PBMC. A preferential expansion of TCR-gamma delta+ cells was also shown after SFMC stimulation with live, but not with heat-killed Yersinia or with TT. We conclude that a common mechanism is involved in the selective expansion of TCR-gamma delta+ lymphocytes upon SFMC infection with live Yersinia or upon contact with 65-kD hsp. Out of a panel of TCR-gamma delta+ T lymphocyte clones (TLC) derived from a human 65-kD hsp-stimulated line, only a minority of TLC proliferated weakly upon restimulation with this antigen in the presence of autologous monocytes, whereas TCR-alpha beta+ TLC responded vigorously to the human 65-kD hsp and in some cases also cross-recognized the mycobacterial hsp homologue and/or heat-killed Yersinia. This implies that additional factors or cells may be present in the milieu of SFMC cultures that propagate the expansion of TCR-gamma delta+ cells in response to 65-kD hsp or live bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonneville M., Janeway C. A., Jr, Ito K., Haser W., Ishida I., Nakanishi N., Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of gamma delta T cells. Nature. 1988 Dec 1;336(6198):479–481. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- Born W., Hall L., Dallas A., Boymel J., Shinnick T., Young D., Brennan P., O'Brien R. Recognition of a peptide antigen by heat shock--reactive gamma delta T lymphocytes. Science. 1990 Jul 6;249(4964):67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Londei M., Jackson A. M., Hercend T., Brenner M. B., Maini R. N., Feldmann M. T cells expressing gamma delta chain receptors in rheumatoid arthritis. J Autoimmun. 1988 Aug;1(4):319–326. doi: 10.1016/0896-8411(88)90002-9. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Dialynas D. P., Strominger J. L., Smith J. A., Owen F. L., Seidman J. G., Ip S., Rosen F., Krangel M. S. Identification of a putative second T-cell receptor. Nature. 1986 Jul 10;322(6075):145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- Bröker B., Lydyard P. M., Emmrich F. The role of gamma delta T cells in the normal and disordered immune system. Klin Wochenschr. 1990 May 17;68(10):489–495. doi: 10.1007/BF01648239. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Bucy R. P., Chen C. L., Cooper M. D. Tissue localization and CD8 accessory molecule expression of T gamma delta cells in humans. J Immunol. 1989 May 1;142(9):3045–3049. [PubMed] [Google Scholar]
- Ciccone E., Ferrini S., Bottino C., Viale O., Prigione I., Pantaleo G., Tambussi G., Moretta A., Moretta L. A monoclonal antibody specific for a common determinant of the human T cell receptor gamma/delta directly activates CD3+WT31- lymphocytes to express their functional program(s). J Exp Med. 1988 Jul 1;168(1):1–11. doi: 10.1084/jem.168.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graeff-Meeder E. R., van der Zee R., Rijkers G. T., Schuurman H. J., Kuis W., Bijlsma J. W., Zegers B. J., van Eden W. Recognition of human 60 kD heat shock protein by mononuclear cells from patients with juvenile chronic arthritis. Lancet. 1991 Jun 8;337(8754):1368–1372. doi: 10.1016/0140-6736(91)93057-g. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Bailey L. C., Bacon P. A. In vitro responses to a 65-kilodalton mycobacterial protein by synovial T cells from inflammatory arthritis patients. J Immunol. 1989 Oct 15;143(8):2494–2500. [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Jenner P. J., Colston M. J., Bacon P. A. Recognition of a mycobacteria-specific epitope in the 65-kD heat-shock protein by synovial fluid-derived T cell clones. J Exp Med. 1990 Mar 1;171(3):831–841. doi: 10.1084/jem.171.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Groh V., Porcelli S., Fabbi M., Lanier L. L., Picker L. J., Anderson T., Warnke R. A., Bhan A. K., Strominger J. L., Brenner M. B. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989 Apr 1;169(4):1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas W., Kaufman S., Martinez C. The development and function of gamma delta T cells. Immunol Today. 1990 Oct;11(10):340–343. doi: 10.1016/0167-5699(90)90133-t. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Havlir D. V., Ellner J. J., Chervenak K. A., Boom W. H. Selective expansion of human gamma delta T cells by monocytes infected with live Mycobacterium tuberculosis. J Clin Invest. 1991 Feb;87(2):729–733. doi: 10.1172/JCI115053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann E., Lohse A. W., Van der Zee R., Van Eden W., Mayet W. J., Probst P., Poralla T., Meyer zum Büschenfelde K. H., Fleischer B. Synovial fluid-derived Yersinia-reactive T cells responding to human 65-kDa heat-shock protein and heat-stressed antigen-presenting cells. Eur J Immunol. 1991 Sep;21(9):2139–2143. doi: 10.1002/eji.1830210923. [DOI] [PubMed] [Google Scholar]
- Hermann E., Mayet W. J., Lohse A. W., Grevenstein J., Meyer zum Büschenfelde K. H., Fleischer B. Proliferative response of synovial fluid and peripheral blood mononuclear cells to arthritogenic and non-arthritogenic microbial antigens and to the 65-kDa mycobacterial heat-shock protein. Med Microbiol Immunol. 1990;179(4):215–224. doi: 10.1007/BF00195252. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Holzmann B., Johnson J. P. A beta-galactosidase linked immunoassay for the analysis of antigens on individual cells. J Immunol Methods. 1983 Jun 10;60(3):359–367. doi: 10.1016/0022-1759(83)90293-4. [DOI] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D., Bender A., Schondelmaier S., Schoel B., Kaufmann S. H. A large fraction of human peripheral blood gamma/delta + T cells is activated by Mycobacterium tuberculosis but not by its 65-kD heat shock protein. J Exp Med. 1990 Mar 1;171(3):667–679. doi: 10.1084/jem.171.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson-Parra A., Söderström K., Ferm M., Ivanyi J., Kiessling R., Klareskog L. Presence of human 65 kD heat shock protein (hsp) in inflamed joints and subcutaneous nodules of RA patients. Scand J Immunol. 1990 Jun;31(6):283–288. doi: 10.1111/j.1365-3083.1990.tb02770.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat-shock proteins: a missing link in the host-parasite relationship? Med Microbiol Immunol. 1990;179(2):61–66. doi: 10.1007/BF00198526. [DOI] [PubMed] [Google Scholar]
- Munk M. E., Gatrill A. J., Kaufmann S. H. Target cell lysis and IL-2 secretion by gamma/delta T lymphocytes after activation with bacteria. J Immunol. 1990 Oct 15;145(8):2434–2439. [PubMed] [Google Scholar]
- Rajasekar R., Sim G. K., Augustin A. Self heat shock and gamma delta T-cell reactivity. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1767–1771. doi: 10.1073/pnas.87.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Res P. C., Schaar C. G., Breedveld F. C., van Eden W., van Embden J. D., Cohen I. R., de Vries R. R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988 Aug 27;2(8609):478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Smith M. D., Bröker B., Moretta L., Ciccone E., Grossi C. E., Edwards J. C., Yüksel F., Colaco B., Worman C., Mackenzie L. T gamma delta cells and their subsets in blood and synovial tissue from rheumatoid arthritis patients. Scand J Immunol. 1990 Dec;32(6):585–593. doi: 10.1111/j.1365-3083.1990.tb03200.x. [DOI] [PubMed] [Google Scholar]
- Stingl G., Koning F., Yamada H., Yokoyama W. M., Tschachler E., Bluestone J. A., Steiner G., Samelson L. E., Lew A. M., Coligan J. E. Thy-1+ dendritic epidermal cells express T3 antigen and the T-cell receptor gamma chain. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4586–4590. doi: 10.1073/pnas.84.13.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderström K., Halapi E., Nilsson E., Grönberg A., van Embden J., Klareskog L., Kiessling R. Synovial cells responding to a 65-kDa mycobacterial heat shock protein have a high proportion of a TcR gamma delta subtype uncommon in peripheral blood. Scand J Immunol. 1990 Nov;32(5):503–515. doi: 10.1111/j.1365-3083.1990.tb03191.x. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel F., Hercend T. Subpopulations of human peripheral T gamma delta lymphocytes. Immunol Today. 1989 Jun;10(6):186–188. doi: 10.1016/0167-5699(89)90321-6. [DOI] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]



