Abstract
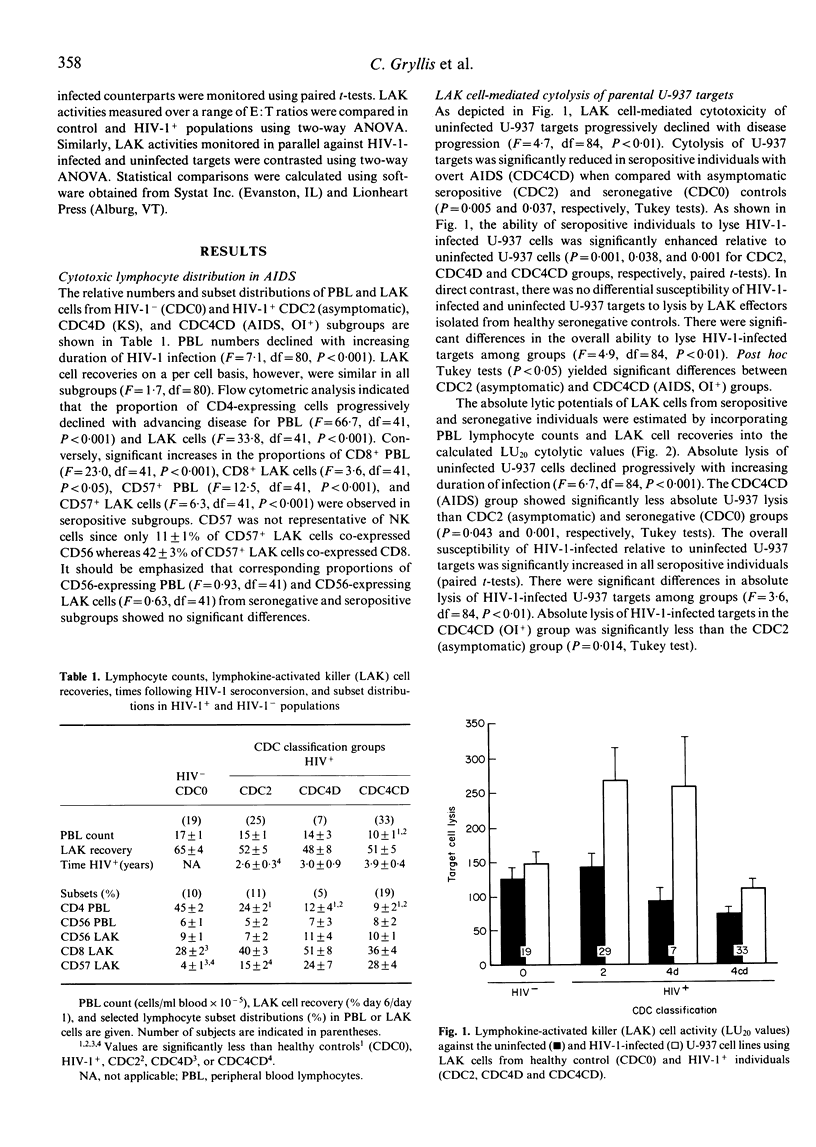
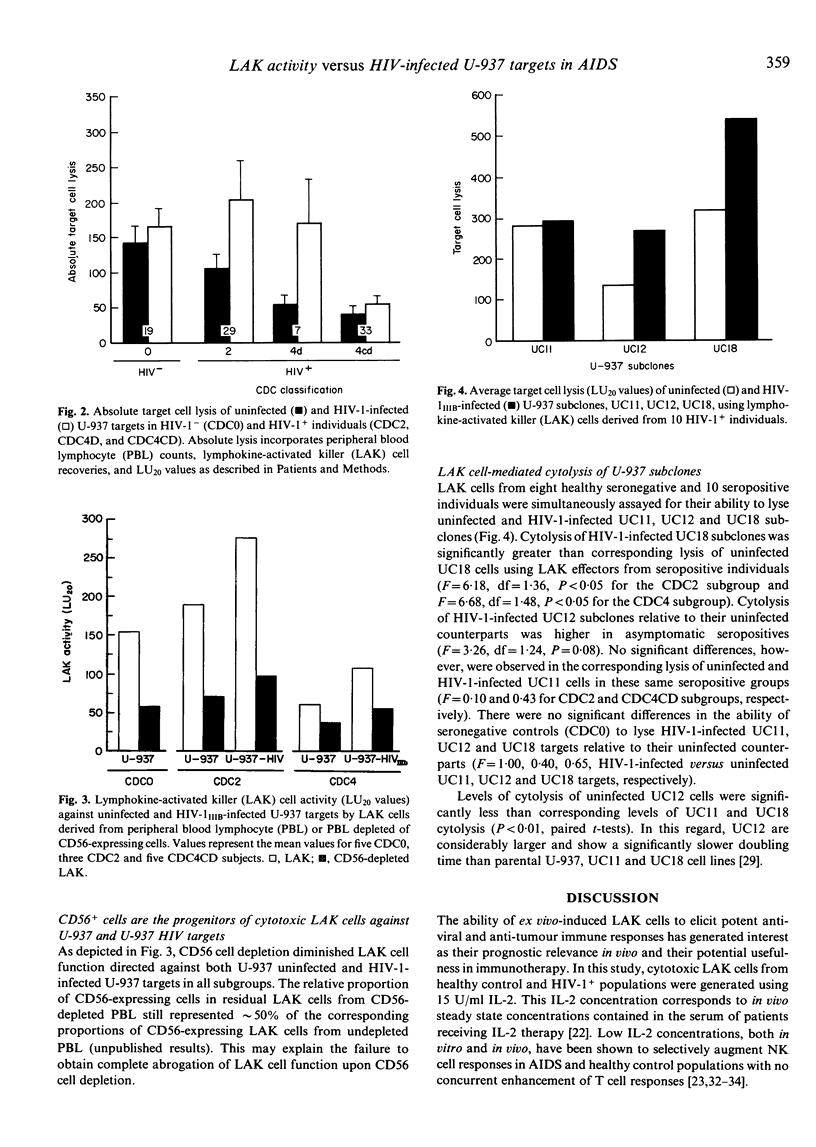
The role of natural killer (NK) cells and their inducible counterparts, lymphokine-activated killer (LAK) cells in AIDS with regard to HIV-1 viral immunosurveillance and the control of secondary opportunistic disease has yet to be established. In this study, we have demonstrated that LAK cells derived from all HIV-1+ groups showed striking increases in their capacity to lyse HIV-1-infected U-937 cells relative to their uninfected U-937 counterparts. Surprisingly, similarly derived LAK cells from healthy seronegative controls showed no differences in their lysis of HIV-1-infected versus uninfected U-937 cells. The differential ability of LAK effectors from seropositive donors to lyse HIV-1-infected targets was demonstrable using a number of U-937 subclones and their HIV-1-infected counterparts. Again, no differences in LAK cell-mediated lysis of HIV-1-infected and uninfected U-937 subclones were observed in seronegative individuals. Our findings that HIV-1+ individuals show selective expansion of non-MHC restricted, HIV-1-directed cytotoxic LAK cells indicate that natural immunity may indeed play a role in HIV-1 viral immunosurveillance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandyopadhyay S., Ziegner U., Campbell D. E., Miller D. S., Hoxie J. A., Starr S. E. Natural killer cell-mediated lysis of T cell lines chronically infected with HIV-1. Clin Exp Immunol. 1990 Mar;79(3):430–435. doi: 10.1111/j.1365-2249.1990.tb08107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulerice F., Geleziunas R., Bour S., Li H. L., D'Addario M., Roulston A., Hiscott J., Wainberg M. A. Differential susceptibilities of U-937 cell clones to infection by human immunodeficiency virus type 1. J Virol. 1992 Feb;66(2):1183–1187. doi: 10.1128/jvi.66.2.1183-1187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. G., Benarrosh S., Margolese R. G. Peripheral blood natural killer cell activity in human breast cancer patients and its modulation by T-cell growth factor and autologous plasma. Cancer. 1986 Aug 15;58(4):895–902. doi: 10.1002/1097-0142(19860815)58:4<895::aid-cncr2820580416>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Brenner B. G., Dascal A., Margolese R. G., Wainberg M. A. Natural killer cell function in patients with acquired immunodeficiency syndrome and related diseases. J Leukoc Biol. 1989 Jul;46(1):75–83. doi: 10.1002/jlb.46.1.75. [DOI] [PubMed] [Google Scholar]
- Brenner B. G., Gryllis C., Gornitsky M., Cupples W., Wainberg M. Differential effects of chemotherapy-induced and HIV-1-induced immunocompromise on NK and LAK activities using breast cancer and HIV-1 seropositive patient populations. Anticancer Res. 1991 Mar-Apr;11(2):969–974. [PubMed] [Google Scholar]
- Brenner B. G., Gryllis C., Wainberg M. A. Role of antibody-dependent cellular cytotoxicity and lymphokine-activated killer cells in AIDS and related diseases. J Leukoc Biol. 1991 Dec;50(6):628–640. doi: 10.1002/jlb.50.6.628. [DOI] [PubMed] [Google Scholar]
- Chehimi J., Bandyopadhyay S., Prakash K., Perussia B., Hassan N. F., Kawashima H., Campbell D., Kornbluth J., Starr S. E. In vitro infection of natural killer cells with different human immunodeficiency virus type 1 isolates. J Virol. 1991 Apr;65(4):1812–1822. doi: 10.1128/jvi.65.4.1812-1822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin T. W., Plaeger-Marshall S., Haas A., Ank B. J., Stiehm E. R. Lymphokine-activated killer cells in primary immunodeficiencies and acquired immunodeficiency syndrome. Clin Immunol Immunopathol. 1989 Dec;53(3):449–459. doi: 10.1016/0090-1229(89)90007-x. [DOI] [PubMed] [Google Scholar]
- Eberlein T. J., Rodrick M. L., Massaro A. F., Jung S. E., Mannick J. A., Schoof D. D. Immunomodulatory effects of systemic low-dose recombinant interleukin-2 and lymphokine-activated killer cells in humans. Cancer Immunol Immunother. 1989;30(3):145–150. doi: 10.1007/BF01669422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Schnittman S. M., Poli G., Koenig S., Pantaleo G. NIH conference. Immunopathogenic mechanisms in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1991 Apr 15;114(8):678–693. doi: 10.7326/0003-4819-114-8-678. [DOI] [PubMed] [Google Scholar]
- Gambacorti-Passerini C., Rivoltini L., Radrizzani M., Belli F., Sciorelli G., Ravagnani F., Galazka A. R., Cascinelli N., Parmiani G. Differences between in vivo and in vitro activation of cancer patient lymphocytes by recombinant interleukin 2: possible role for lymphokine-activated killer cell infusion in the in vivo-induced activation. Cancer Res. 1989 Sep 15;49(18):5230–5234. [PubMed] [Google Scholar]
- Grimm E. A. Human lymphokine-activated killer cells (LAK cells) as a potential immunotherapeutic modality. Biochim Biophys Acta. 1986 Dec 17;865(3):267–279. doi: 10.1016/0304-419x(86)90017-x. [DOI] [PubMed] [Google Scholar]
- Gryllis C., Wainberg M. A., Gornitsky M., Brenner B. Diminution of inducible lymphokine-activated killer cell activity in individuals with AIDS-related disorders. AIDS. 1990 Dec;4(12):1205–1212. doi: 10.1097/00002030-199012000-00004. [DOI] [PubMed] [Google Scholar]
- Katz J. D., Mitsuyasu R., Gottlieb M. S., Lebow L. T., Bonavida B. Mechanism of defective NK cell activity in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. II. Normal antibody-dependent cellular cytotoxicity (ADCC) mediated by effector cells defective in natural killer (NK) cytotoxicity. J Immunol. 1987 Jul 1;139(1):55–60. [PubMed] [Google Scholar]
- Kedar E., Rezai A. R., Giorgi J. V., Gale R. P., Champlin R. E., Mitsuyasu R. T., Fahey J. L. Immunomodulating effects in vitro of interleukin-2 and interferon-gamma on human blood and bone marrow mononuclear cells. Nat Immun Cell Growth Regul. 1988;7(1):13–30. [PubMed] [Google Scholar]
- Landay A., Ohlsson-Wilhelm B., Giorgi J. V. Application of flow cytometry to the study of HIV infection. AIDS. 1990 Jun;4(6):479–497. doi: 10.1097/00002030-199006000-00001. [DOI] [PubMed] [Google Scholar]
- Ljunggren K., Karlson A., Fenyö E. M., Jondal M. Natural and antibody-dependent cytotoxicity in different clinical stages of human immunodeficiency virus type 1 infection. Clin Exp Immunol. 1989 Feb;75(2):184–189. [PMC free article] [PubMed] [Google Scholar]
- McMannis J. D., Fisher R. I., Creekmore S. P., Braun D. P., Harris J. E., Ellis T. M. In vivo effects of recombinant IL-2. I. Isolation of circulating Leu-19+ lymphokine-activated killer effector cells from cancer patients receiving recombinant IL-2. J Immunol. 1988 Feb 15;140(4):1335–1340. [PubMed] [Google Scholar]
- Melder R. J., Balachandran R., Rinaldo C. R., Gupta P., Whiteside T. L., Herberman R. B. Cytotoxic activity against HIV-infected monocytes by recombinant interleukin 2-activated natural killer cells. AIDS Res Hum Retroviruses. 1990 Aug;6(8):1011–1015. doi: 10.1089/aid.1990.6.1011. [DOI] [PubMed] [Google Scholar]
- Nixon D. F., McMichael A. J. Cytotoxic T-cell recognition of HIV proteins and peptides. AIDS. 1991 Sep;5(9):1049–1059. [PubMed] [Google Scholar]
- Ortaldo J. R., Longo D. L. Human natural lymphocyte effector cells: definition, analysis of activity, and clinical effectiveness. J Natl Cancer Inst. 1988 Sep 7;80(13):999–1010. doi: 10.1093/jnci/80.13.999. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A., Overton R. Lymphokine-activated killer cells. Analysis of progenitors and effectors. J Exp Med. 1986 Oct 1;164(4):1193–1205. doi: 10.1084/jem.164.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki A., Nakano H., Minato K., Nakagawa K., Sasaki Y., Saijo N. The difference in surface phenotypes between cytotoxic lymphocytes induced in vivo by systemic administration of human recombinant interleukin-2 and lymphokine activated killer cells induced in vitro. Eur J Cancer Clin Oncol. 1988 Jun;24(6):1055–1060. doi: 10.1016/0277-5379(88)90159-9. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Gemlo B. T., Myers W. W., Rayner A. A., Lanier L. L. In vivo and in vitro activation of natural killer cells in advanced cancer patients undergoing combined recombinant interleukin-2 and LAK cell therapy. J Clin Oncol. 1987 Dec;5(12):1933–1941. doi: 10.1200/JCO.1987.5.12.1933. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986 Sep 1;164(3):814–825. doi: 10.1084/jem.164.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pross H. F., Baines M. G., Rubin P., Shragge P., Patterson M. S. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981 Jan;1(1):51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- Rappocciolo G., Toso J. F., Torpey D. J., 3rd, Gupta P., Rinaldo C. R., Jr Association of alpha interferon production with natural killer cell lysis of U937 cells infected with human immunodeficiency virus. J Clin Microbiol. 1989 Jan;27(1):41–48. doi: 10.1128/jcm.27.1.41-48.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz J., Schmidt R. E., Michon J., Hercend T., Schlossman S. F. Characterization of functional surface structures on human natural killer cells. Adv Immunol. 1988;42:181–211. doi: 10.1016/s0065-2776(08)60845-7. [DOI] [PubMed] [Google Scholar]
- Riviere Y., Tanneau-Salvadori F., Regnault A., Lopez O., Sansonetti P., Guy B., Kieny M. P., Fournel J. J., Montagnier L. Human immunodeficiency virus-specific cytotoxic responses of seropositive individuals: distinct types of effector cells mediate killing of targets expressing gag and env proteins. J Virol. 1989 May;63(5):2270–2277. doi: 10.1128/jvi.63.5.2270-2277.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. J., Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990 Dec 15;76(12):2421–2438. [PubMed] [Google Scholar]
- Robinson W. E., Jr, Mitchell W. M., Chambers W. H., Schuffman S. S., Montefiori D. C., Oeltmann T. N. Natural killer cell infection and inactivation in vitro by the human immunodeficiency virus. Hum Pathol. 1988 May;19(5):535–540. doi: 10.1016/s0046-8177(88)80200-4. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Mikovits J. A., Kalyanaraman V. S., Overton R., Stevenson H., Stromberg K., Herberman R. B., Farrar W. L., Ortaldo J. R. Analysis of effector mechanisms against HTLV-I- and HTLV-III/LAV-infected lymphoid cells. J Immunol. 1986 May 15;136(10):3619–3624. [PubMed] [Google Scholar]
- Schwartz D. H., Merigan T. C. Interleukin-2 in the treatment of HIV disease. Biotherapy. 1990;2(2):119–136. doi: 10.1007/BF02173452. [DOI] [PubMed] [Google Scholar]
- Sirianni M. C., Soddu S., Malorni W., Arancia G., Aiuti F., Soddus S. Mechanism of defective natural killer cell activity in patients with AIDS is associated with defective distribution of tubulin. J Immunol. 1988 Apr 15;140(8):2565–2568. [PubMed] [Google Scholar]
- Skibber J. M., Lotze M. T., Muul L. M., Uppenkamp I. K., Ross W., Rosenberg S. A. Human lymphokine-activated killer cells: further isolation and characterization of the precursor and effector cell. Nat Immun Cell Growth Regul. 1987;6(6):291–305. [PubMed] [Google Scholar]
- Tremblay M., Wainberg M. A. Susceptibility to AZT of HIV-1 variants grown in Epstein-Barr virus-transformed B cell lines. J Infect Dis. 1989 Jul;160(1):31–36. doi: 10.1093/infdis/160.1.31. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. S., Stanley S. D., Nastala C. A., Austin A. A., Bartlett J. A., Stine K. C., Lyerly H. K., Bolognesi D. P., Weinhold K. J. Alterations in antibody-dependent cellular cytotoxicity during the course of HIV-1 infection. Humoral and cellular defects. J Immunol. 1990 May 1;144(9):3375–3384. [PubMed] [Google Scholar]
- Vuillier F., Bianco N. E., Montagnier L., Dighiero G. Selective depletion of low-density CD8+, CD16+ lymphocytes during HIV infection. AIDS Res Hum Retroviruses. 1988 Apr;4(2):121–129. doi: 10.1089/aid.1988.4.121. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Plata F. Cytotoxic T lymphocytes against HIV. AIDS. 1990 Mar;4(3):177–184. doi: 10.1097/00002030-199003000-00001. [DOI] [PubMed] [Google Scholar]
- Weil-Hillman G., Fisch P., Prieve A. F., Sosman J. A., Hank J. A., Sondel P. M. Lymphokine-activated killer activity induced by in vivo interleukin 2 therapy: predominant role for lymphocytes with increased expression of CD2 and leu19 antigens but negative expression of CD16 antigens. Cancer Res. 1989 Jul 1;49(13):3680–3688. [PubMed] [Google Scholar]
- Weinhold K. J., Lyerly H. K., Matthews T. J., Tyler D. S., Ahearne P. M., Stine K. C., Langlois A. J., Durack D. T., Bolognesi D. P. Cellular anti-GP120 cytolytic reactivities in HIV-1 seropositive individuals. Lancet. 1988 Apr 23;1(8591):902–905. doi: 10.1016/s0140-6736(88)91713-8. [DOI] [PubMed] [Google Scholar]
- Weinhold K. J., Lyerly H. K., Stanley S. D., Austin A. A., Matthews T. J., Bolognesi D. P. HIV-1 GP120-mediated immune suppression and lymphocyte destruction in the absence of viral infection. J Immunol. 1989 May 1;142(9):3091–3097. [PubMed] [Google Scholar]



