Abstract
The intestinal peptide guanylin regulates the electrolyte/water transport in the gastrointestinal epithelium by paracrine/luminocrine mechanisms. Because guanylin also circulates in the blood, we investigated the rat hypothalamo-pituitary region for expression and cellular localization of this peptide. Reverse transcriptase–PCR analyses with guanylin-specific primers revealed expression of the peptide in the pars tuberalis and pars distalis of the pituitary. Western blotting analyses in hypophyseal tissue extracts identified the expected 12.5-kDa immunoreactive peptide by using two different region-specific guanylin antisera. Light and electron microscopic immunocytochemistry with the same antisera localized guanylin in “pars tuberalis-specific cells” in the juxtaneural pars tuberalis adjacent to nerve endings and blood vessels of the hypothalamo-pituitary portal system and in gonadotrophic cells within the distal pars tuberalis and ventrolateral part of the pars distalis. The presence and cell-specific localization of guanylin within the hypothalamo-hypophyseal system indicate that this peptide may be specifically involved in paracrine and endocrine regulatory mechanisms.
Keywords: guanylate cyclase C-activating peptides, pituitary gland, regulatory peptides, gonadotrophic cells
The peptide guanylin, originally extracted and purified from rat small intestine (1), has high sequence and structural homology with Escherichia coli heat-stable enterotoxins (STa) known to cause secretory diarrhea by activation of the guanylate cyclase C receptor (1). Actually, guanylate cyclase C represents the genuine receptor for the endogenous ligand guanylin (2). Cloning of guanylin cDNA from intestinal cDNA libraries revealed that this peptide is synthesized as a 115-amino acid precursor that contains at the C terminus the 15-amino acid peptide isolated from the intestinal extracts (3). Since the discovery of uroguanylin (4) and lymphoguanylin (5), it became evident that guanylin belongs to a family of related peptides. Through activation of guanylate cyclase C, guanylin induces the increase of intracellular cGMP concentration, which mediates phosphorylation and activation of the cystic fibrosis transmembrane conductance regulator Cl− channel (1, 6). Although the function of lymphoguanylin remains to be clarified in detail, guanylin and uroguanylin are obviously involved in electrolyte/water secretion in various ion-transporting epithelia (6). Guanylin and uroguanylin have been localized in paracrine/luminocrine-secreting epithelial cells of the gastrointestinal tract (3, 7–12) and of the small airways (13). On the other hand, a circulating form of guanylin in considerable concentrations has been identified in plasma (14), indicating that endocrine cells and organs may be another source of guanylin. Therefore, in the present study, the rat adenohypophysis was investigated for expression and cellular localization of guanylin in the pars distalis (PD) and pars tuberalis (PT). The latter pituitary region consists of two to four thin layers of epithelial cell cords, often arranged in follicles, closely adherent to the ventral surface of the median eminence of the hypothalamus and the tubero-infundibular region that continues caudally with the pituitary stalk. The distinctive feature in this region is a rich blood capillary network representing the primary plexus of the hypothalamo-pituitary portal system. Studies investigating the functional role of the PT cells have assigned them the putative role of intervening on the liquid traffic between the hypothalamo-adenohypophyseal portal vessels and the cerebrospinal fluid toward the subarachnoidal space and the third ventricle (15, 16). Our study identifies the presence of guanylin and its cell-specific localization in different regions of the rat adenohypophysis, implicating the respective cells in paracrine and endocrine regulatory functions.
Materials and Methods
Reverse Transcriptase–PCR (RT-PCR) Analysis.
Male Sprague–Dawley rats (n = 5) weighing 250–300 g (Harlan–Sprague–Dawley) were anesthetized and killed by cervical dislocation. The whole pituitary gland, the tubero-infundibular region with the pituitary PT, and the duodenum (positive control) were removed. For total RNA isolation, 25-mg tissue pieces were frozen in liquid nitrogen together with 600 ml of homogenization buffer including 40 μl/ml 2-mercaptoethanol, disrupted, and homogenized by 5 min of shaking at 2,000 rpm in a bead mill (Braun, Melsungen, Germany). After elution of the RNA from the columns with RNase-free water, its concentration and purity were analyzed spectrophotometrically (260/280 nm). The integrity of RNA preparations was analyzed by gel electrophoresis on ethidium bromide-stained agarose-formaldehyde gels (17). In a 22-μl reaction volume, 2.5 μg of total RNA was incubated with 200 pmol oligo(dT) 15 primer (Promega) for 10 min at 70°C. RNAs were reverse transcribed by adding 400 units of M-MLV Reverse Transcriptase-RNase H Minus (Promega)/80 units of RNase Inhibitor (Promega)/0.5 mM each dNTP/50 mM Tris⋅HCl, pH 8.3/75 mM KCl/3 mM MgCl2/10 mM DTT to a total volume of 40 μl (18). The reaction sample was incubated for 90 min at 37°C and stored at −20°C. For the reverse transcriptase reaction, primer pairs specific for rat guanylin were deduced from the GenBank cDNA sequences (accession no. M93005) by using primer design and alignment software (ref. 19 and www.genome.wi.mit.edu/genome_software/other/primer.html) and checked for specificity and homology. The oligonucleotide primers were obtained from MWG Biotec (Ebersberg, Germany). According to the published protocol (20), the primer pair for guanylin (forward 5′-CCA GAG CTA TGT AGC CAA TC; reverse 5′-CAG TAG CTC CAT TTA TTG ACA GAG) amplifies a 326-bp fragment from nucleotides 208 to 533. The integrity of the different transcribed cDNAs was checked by using the primer pairs (forward 5′-ATG GGA AGC TGG TCA TCA AC; reverse 5′-CCA CAG TCT TCT GAG TGG CA) specific for the rat gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), amplifying a 375-bp fragment from nucleotides 256 to 630 of the GAPDH cDNA sequence (GenBank accession no. X02231). The PCR-master mixture consisted of 5 μl of 10× PCR buffer [160 mM (NH4)2SO4/500 mM Tris⋅HCl, pH 8.8/0.1% Tween 20], 2 mM MgCl2, 2 units of Taq-polymerase (PanSystems, Nürnberg, Germany), 200 nM each dNTP, 100 pmol of each primer, and 2 μl of cDNA in a final volume of 50 μl. Target DNA samples were amplified on a Touchdown Thermocycler (Hybaid, Heidelberg, Germany). After an initial denaturation of 94°C for 4 min, reactions were subjected to 35 cycles of the following thermal program: 94°C for 30 s, 60°C for 1 min for guanylin (57°C for 1 min for GAPDH), and 72°C for 30 s; this program was followed by a final 5-min elongation step at 72°C. Amplification products were run on an ethidium bromide-stained 1.8% 89 mM Tris/89 mM boric acid/2 mM EDTA (pH 8.3) agarose gel.
Western Blot Analysis.
Male Sprague–Dawley rats (n = 10; body weight of 250–300 g) were anesthetized and killed by cervical dislocation. Tissue specimens from hypophysis, duodenum, and colon (the last two used as positive controls) were excised and rinsed repeatedly in ice-cold saline. After boiling in 1 M acetic acid for 10 min, the tissues were homogenized with an Ultra-Turrax homogenizer (Janke & Kunkel, Staufen, Germany). The homogenates were centrifuged at 20,000 × g for 20 min at 4°C and extracted by applying them to an octadecasilyl (C18) Sep-Pak cartridge (Waters) as described (8). The tissue extracts were separated by Tricine-SDS/PAGE (21) in 16.5% polyacrylamide gels and electroblotted onto hydrophobic poly(vinylidene difluoride)-based membranes (Pall) as described (8, 13). The membranes were incubated overnight at 4°C with the guanylin antisera K42 and K605 (see below), each in a 1:1,500 dilution. Immunoreactive polypeptides were visualized after incubation with alkaline phosphatase-conjugated goat anti-rabbit IgG (1:15,000; Sigma), with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate as chromogens (Sigma).
Immunohistochemistry.
Animals and tissue preparation.
Adult Sprague–Dawley rats (n = 30; body weight 250–300 g) of different ages and both sexes were perfused under deep anesthesia with sodium pentobarbital (50 mg/kg, i.p.; Nembutal, Abbott) via the left ventricle with 150 ml of 0.01 M PBS (pH 7.3), followed by 300 ml of a cold fixative mixture [PAF: 4% (vol/vol) paraformaldehyde/0.2% picric acid in 0.1 M phosphate buffer, pH 7.3] containing 0.25% glutaraldehyde. Pituitary glands and the hypothalamic region between the optic chiasma and the mammillary bodies, including the pituitary stalk, were quickly excised and postfixed for 48 h with the same cold PAF mixture without glutaraldehyde; thereafter, they were divided into four groups that were, respectively, (i) dehydrated, paraffin-embedded, and cut (Biocut, Reichert-Jung, Wien, Austria) into 7-μm-thick sections that were mounted on albumin-coated slides; (ii) thoroughly washed and cryoprotected with cold 0.1 M phosphate buffer (pH 7.3) containing 15% (vol/vol) sucrose and then frozen and cut (Frigocut, Reichert-Jung) into 15-μm-thick sections that were either mounted on gelatin chrome-alum-coated slides or treated as free-floating sections; (iii) cut with a vibratome (Microslicer, D.S.K. Dosaka EM, Kyoto, Japan) into 60-μm-thick sections that were processed for free-floating incubations and preembedding immunoelectron microscopy; or (iv) cut in small tissue blocks, routinely embedded in epoxy resin and cut in serial semithin (0.5-μm) sections.
Antibodies.
Two region-specific rabbit antisera against guanylin that have been raised against guanylin-(34–46) (antiserum K42) and guanylin-(101–115) (antiserum K605) were used as detailed (8); both antisera have been characterized and used in previous studies (8, 13). Moreover, a panel of antisera was employed for identification and characterization of guanylin immunoreactive cells: anti-human growth hormone (lot AFP872189); anti-rat prolactin (lot AFPC2381); anti-human luteinizing hormone (h-β-LH, lot AFP54372); anti-human thyroid stimulating hormone (h-β-TSH, lot AFP62423473); and anti-human chorionic gonadotropin (lot AFP310784)—all from the National Hormone and Pituitary Program of The National Institute of Arthritis, Diabetes, and Digestive and Kidney Diseases (Baltimore, MD). Anti-human adrenocorticotrophic hormone (IHC8740) and anti-met-enkephalin (IHC8602) were from Peninsula Laboratories; anti-β-endorphin (RPN1622) was from Amersham Pharmacia; anti-glial fibrillary acid protein (GFAP; Z334/015) and anti-protein S100 (Z 311/129) were from Dakopatts (Copenhagen). Furthermore, three monoclonal antibodies against chromogranins A, B, and C (kindly donated by R. Buffa, Institute of Morbid Anatomy, University of Milan) were used as endocrine cell markers.
Immunohistochemical protocol.
Paraffin-sections were dewaxed with toluene, and semithin plastic sections were dewaxed with sodium ethoxide [3% (vol/vol) NaOH in absolute ethanol]. Rehydrated sections were pretreated with 0.1 M PBS containing 0.3% H2O2 to block endogenous peroxidase activity (22). After preincubation with normal goat serum diluted 1:30 with PBS/BSA (PBS containing 1% BSA, Sigma), the sections were incubated in a moist chamber for 4–7 days at 4°C with the guanylin antisera K42 and K605, and consecutive sections were incubated with antisera against the various peptides and proteins used for identification and characterization of the respective cells. All antisera and antibodies were diluted in PBS/BSA containing 0.3% Triton X-100 (PBST; Merck) at 1:2,500 for paraffin- or plastic-embedded sections and at 1:20,000 to 1:40,000 for free-floating sections. A long incubation time, i.e., 1 week, allowed us to use the highest dilutions of primary antisera and to avoid any background. The sections were then incubated for 2 h at room temperature with biotinylated goat anti-rabbit IgG or horse anti-mouse IgG (Vector Laboratories) diluted 1:2,000 with PBST and subsequently for 1 h at room temperature with avidin-biotin-peroxidase complex (ABC Elite, Vector Laboratories) diluted 1:4,000 with PBST. The peroxidase activity was then visualized by incubation of the sections for 3 min in the enzyme reaction mixture containing 0.04% 3–3′ diaminobenzidine tetrahydrochloride (Fluka, Deisenhofen, Germany), 0.4% nickel ammonium sulfate, and 0.003% H2O2 in 0.05M Tris⋅HCl buffer (pH 7.6).
Immunoelectron microscopy.
Free-floating 60-μm-thick vibratome sections of tubero-infundibular PT specimens stored for 48–72 h at 4°C in 0.1 M PBS containing 0.08–0.1% Triton X-100 were preincubated with normal goat serum (1:30) and then incubated for 1 week at 4°C with K605 and K42 antisera diluted 1:15,000 with PBS containing 0.1% Triton X-100. After washing with PBS, the sections were incubated overnight at 4°C with 1-nm gold-conjugated goat anti-rabbit IgG (Nanogold, Amersham Pharmacia) diluted 1:50 with PBS, washed repeatedly with PBS, and submitted to a silver intensifying reaction (20 min at 20°C) by using the kit supplied by Amersham Pharmacia. The sections were then washed several times in bidistilled water and a final bath in PBS. The specimens were treated with a reduced osmium solution [1% osmium tetroxide/1.5% (vol/vol) potassium ferrocyanide in bidistilled water] for 1 h on a crushed ice bath; subsequently, they were dehydrated in ascending ethanols and propylene oxide and flat-embedded in epoxy resin (Luweak 812, Nakarai Chemical, Kyoto) on siliconized slides and coverslipped. The immunopositive areas were selected under light microscope, then squared, glued on epoxy resin blocks, and cut in an ultramicrotome (Leica, Wetzlar, Germany) and mounted on collodion-coated grids. After the usual counterstains with uranyl acetate and lead citrate, the sections were examined under an electron microscope.
Specificity controls.
Method- and antibody-specificity controls included the replacement of primary antisera either by PBST alone or by normal rabbit serum in the immunohistochemical staining protocol and the preabsorption of the primary antisera with an excess of antigens (10–25 μg of antigen per ml of antiserum in working dilution) for 24–48 h at 4°C. All controls confirmed the specificity of immunostainings.
Results
Expression of Guanylin in the Adenohypophysis.
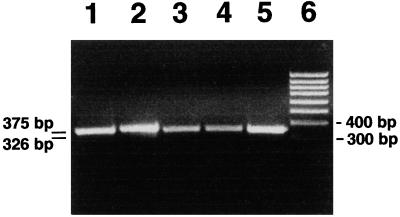
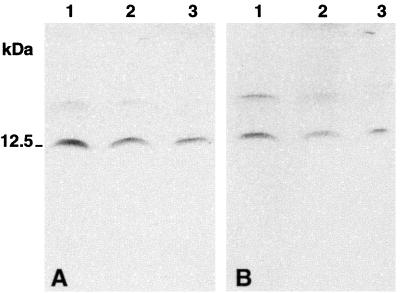
RT-PCR analysis with rat proguanylin-specific oligonucleotide primers yielded a 326-bp expected transcript in the tubero-infundibular PT as well as in the whole hypophysis. This transcript signal completely matched that obtained from rat duodenal specimens used as a positive control (Fig. 1). In Western blot analyses, both guanylin antisera (K42, K605) coincidentally identified a major immunoreactive band of ≈12.5 kDa molecular mass in extracts of rat whole hypophysis, corresponding to the molecular mass of guanylin (3). This hypophyseal peptide comigrated with the immunoreactive bands also recognized by the antisera K42 and K605 in homogenates of the rat duodenum and colon that were used as positive controls (Fig. 2). In all extracts both antisera also identified a faintly stained band at ≈17 kDa.
Figure 1.
RT-PCR analysis with guanylin-specific primers shows amplification products of correct size in rat whole hypophysis, PT of the hypophysis, and duodenum; 15 μl of RT-PCR products were loaded on a 1.8% 89 mM Tris/89 mM boric acid/2 mM EDTA (pH 8.3) agarose gel containing ethidium bromide. (Lane 1) PT, GAPDH; (lane 2) hypophysis, GAPDH; (lane 3) PT, guanylin; (lane 4) hypophysis, guanylin; (lane 5) duodenum, guanylin; (lane 6) 100-bp marker.
Figure 2.
Western blot after Tricine-SDS/PAGE of rat tissue extracts immunostained with antiserum K42 (A) and antiserum K605 (B). (Lane 1) duodenum; (lane 2) colon; (lane 3) hypophysis. Note the predominant immunoreactive band of ≈12.5 kDa in the rat hypophysis, duodenum, and colon (positive control). The tissue extraction procedure yielded a faintly immunoreactive band at 17 kDa. The low molecular mass markers from Novex (San Diego, CA) were used for the molecular mass calibration.
Cellular and Subcellular Localization.
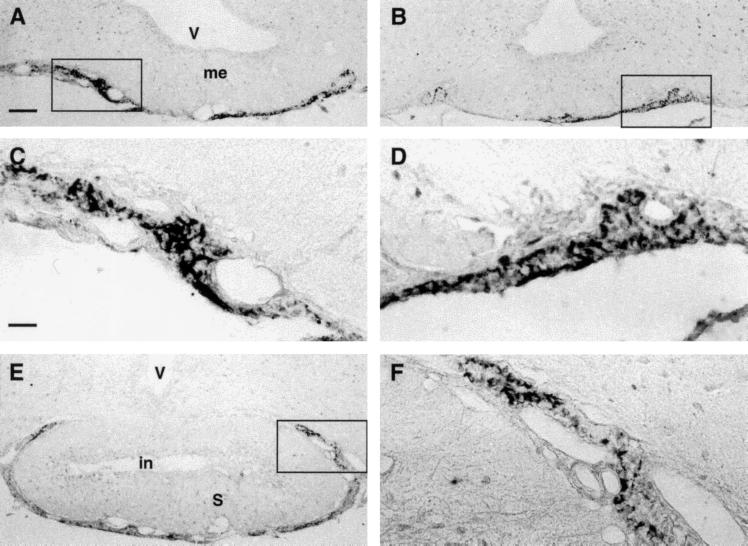
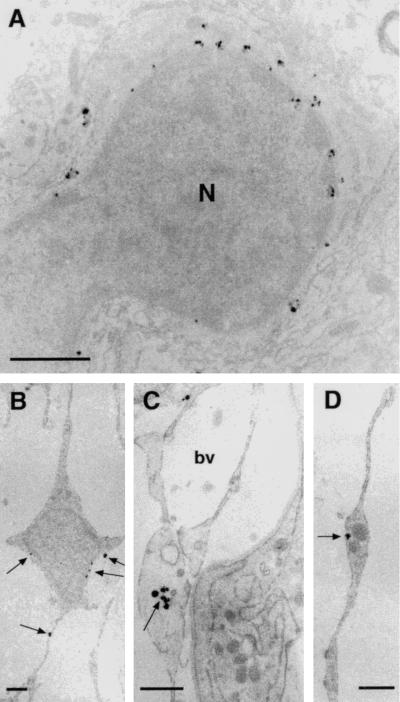
Both guanylin antisera displayed distinct immunoreactivities in the PT and PD of the rat adenohypophysis. We studied the whole PT in its various portions in serial rostrocaudal coronal sections of tubero-infundibular PT specimens. The most rostral sections, the so-called juxtaneural PT, contained some fibrous- or rod-like structures filled with granular material specifically immunostained by both K605 and K42 antisera (Fig. 3 A–D). Similar structures occupied the PT surrounding the pituitary stalk (Fig. 3 E–F). All of these guanylin-immunoreactive structures were irregularly intermingled among the guanylin-unreactive endocrine cells and the blood vessels of this region. Of note, gonadotrophs and thyrotrophs (identified by the antisera against h-β-LH and h-β-TSH, respectively) that mainly occupied this region lacked any immunoreactivity for guanylin. Moreover, series of consecutive sections alternatively immunostained for guanylin and for the various peptides or proteins by the respective antisera revealed that none of these antisera, including anti-GFAP and anti-protein S 100, coincidentally immunostained the guanylin-immunoreactive structures in the rostral PT (data not shown). To characterize these guanylin-immunoreactive structures in detail, we examined the specimens of the rostral PT by preembedding immunoelectron microscopy. Positive gold-silver-intensified deposits lay mainly within particular a cell type characterized by a small ovoid or spherical cell body that branched into thin and sometimes widely extended cytoplasmic elongations. The deposits were contained within small round vesicles (average diameter of 107 ± 7.3 nm) scattered both within the perinuclear cytoplasm of their cell body and within their elongations (Fig. 4). Some of these vesicles contacted the inner surface of the cell membrane and occasionally gave rise to exocytosis-like figures. The cell elongations were often found also around the wall of the rich capillary network of this region (Fig. 4C) or, more rarely, in contact with some nerve fibers (data not shown). None of the numerous cells filled with endocrine secretory granules, normally present in this region, displayed gold-silver deposits.
Figure 3.
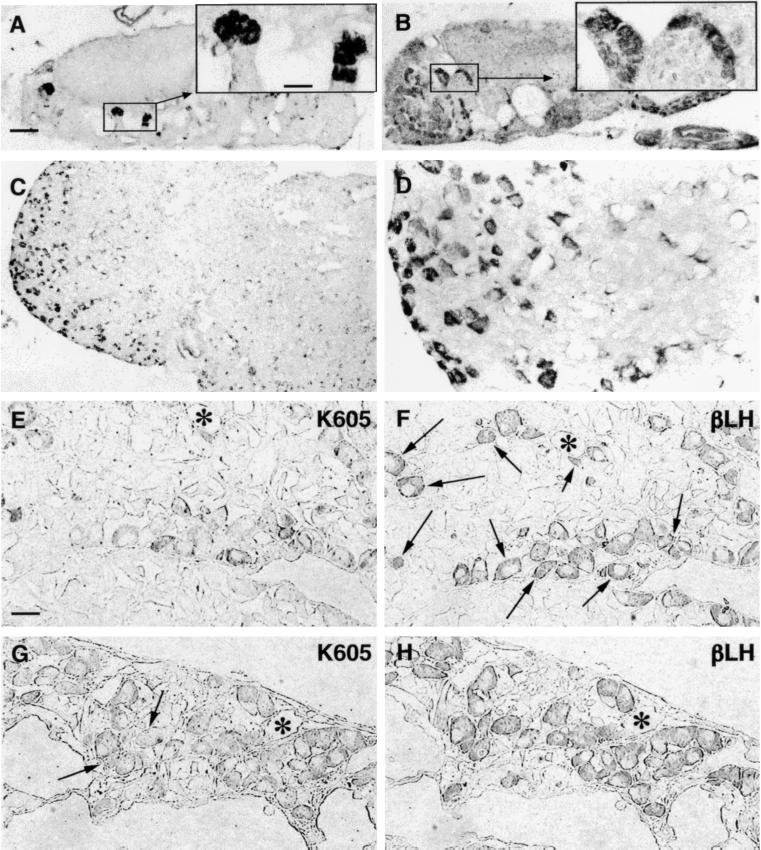
(A and B) Panoramic overview of two neighboring sections of juxtaneural PT region to compare the distribution of guanylin immunoreactivities obtained with the antisera K605 (A) and K42 (B). (C and D) Higher magnification of boxed areas in A and B, respectively. The immunoreactive elements showed a stick- or rod-like morphology. (E) Guanylin immunoreactive (K605) elements in PT at the pituitary stalk level. (F) Higher magnification of the boxed area in E. V, third ventricle; me, median eminence; in, infundibular recess; S, pituitary stalk. (A, B, and E, bar = 100 μm; C, D, and F, bar = 25 μm.)
Figure 4.
(A–D) Ultrathin sections of juxtaneural PT previously submitted to preembedding gold-silver immunocytochemical procedures with K605 antiserum. Gold-silver-enhanced particles are contained in small vesicles both in the cell body and in the cell elongations (arrows). N, nucleus of a specific cells of PT; bv, blood vessel. (Bars = 1 μm.)
In contrast to the pattern of guanylin immunoreactivity in the rostral PT, in the distal portion of the hypophyseal PT (Fig. 5 A and B) and in the PD (Fig. 5 C and D), various frankly cuboid or ovoid cells whose cytoplasm contained granular material were detected that showed guanylin immunoreactivity by the antisera K42 and K605. These cells, mostly arranged in clusters, were located always in the same region of the gland, namely the ventrolateral part of the PD. Morphologically, the majority (about 90%) were large, spherical or cuboid cells, devoid of elongations, with a central nucleus and cytoplasm regularly filled with positive granular material. In contrast, some few and scattered cells were smaller, triangular or irregularly shaped, with two or more thin and short cytoplasmic elongations filled with positive granular material. To characterize these cell types in detail, we immunostained a series of consecutive semithin (0.5 μm) sections alternatively with guanylin antisera and with the panel of antisera against various peptides and proteins. The majority (about 90%) of the guanylin-immunoreactive cells were immunoreactive for h-β-LH (Fig. 5 E–H), and only a few were also immunoreactive for h-β-TSH. The panel of antisera against various peptides and proteins and the antibodies against the chromogranins under study displayed no immunoreactivities in these cells.
Figure 5.
(A and B) Clusters of guanylin immunoreactive cells can be observed in two distal PT sections at different craniocaudal level. (Insets) Higher magnification of boxed areas. (C) Guanylin immunoreactive cells in PD assumed the morphology of the endocrine cells of this region. (D) Higher magnification of an area in C. (E–H) Pair of two consecutive semithin (0.5-μm) sections of PD immunostained for guanylin (K605; E and G) and h-β-LH (βLH; F and H). Almost all guanylin immunoreactive cells show coincident immunoreactivity for h-β-LH, but the number of colocalizing gonadotrophs varies: about one-third in E and F (arrows) and almost all in G and H. Arrows in G indicate the only two guanylin unreactive gonadotrophs. Asterisks label some landmarks to allow the alignment of consecutive sections. (A–C, bar = 100 μm; Insets and E–H, bar = 25 μm.)
Discussion
Using different techniques, we clearly show that guanylin is present in the rat adenohypophysis. RT-PCR analyses identified the expression of guanylin in both the tubero-infundibular PT and the whole hypophysis, which were prepared and separated by microdissection. The same transcript signal was obtained in the rat duodenum used as positive control. Although immunoblotting analyses in the tubero-infundibular PT could not be performed (because of the small amount of the tissue), the presence of guanylin at the translational level in hypophyseal extracts was shown by Western blotting analyses with two different guanylin antisera (K42 and K605) raised against the midportion and the C terminus of the peptide. Thus, both guanylin antisera coincidentally identified a major immunoreactive band of ≈12.5 kDa molecular mass in hypophyseal and intestinal (duodenum and colon) extracts, corresponding to the molecular mass of guanylin (3). In all extracts, however, both antisera also yielded a faintly stained band that may be due to a related protein of higher molecular mass (≈17 kDa). Immunocytochemical studies localized guanylin in distinct cells in the juxtaneural zone of PT, in the distal PT, and in the ventrolateral part of the PD. Despite a common immunoreactivity pattern, guanylin was localized in different cell types in these regions.
The caudal zone of PT and the ventrolateral part of PD contained a distinct number of guanylin-immunoreactive cells that were apparently intermingled into the endocrine cell cords. Consecutive semithin sections alternatively immunostained for guanylin and for various hormones revealed that guanylin immunoreactivity is confined to gonadotrophic cells. Only a few thyrotrophic cells also showed immunoreactivity for this peptide. Although the function of guanylin in these cells is not yet known, it is reasonable to assume that guanylin is released by these cells into the circulation where the peptide has already been identified (14). Judging from the RT-PCR and Western blotting data, it is obvious that the hypophysis is one source of circulating guanylin.
Other than in the distal PT and ventrolateral part of the PD, in the juxtaneural part of the PT, guanylin was confined to cells that were unreactive for other hormones, chromogranins, GFAP, and protein S 100. Using light and electron microscopy, we identified the guanylin immunoreactive cell type found in the juxtaneural part of the PT as the so-called “chromophobic-specific cells of PT” (23, 24). Their characteristic feature is the small cell body with thin cytoplasmic extensions that reach the wall of the blood vessel, some nerve endings, and the pia mater (23). Despite the fact that the PT-specific cells variably display immunoreactivities for α-chain and β-subunits of TSH that depend on the species investigated, the search for a PT-specific hormonal product is still continuing (25). In the present study, both K42 and K605 antisera localized guanylin in these cells, which in consecutive sections proved to be unreactive toward the panel of antisera against various peptides and proteins. In particular, the lack of immunostaining with anti-GFAP and anti-protein S 100 allowed us to exclude the possibility that these cells might be interpreted, at the light microscopic level, as glial cells or follicle-stellate cells, respectively. In accordance with the RT-PCR data, it is quite conceivable that guanylin is produced by the respective cells and may be considered as a potential regulator in this hypophyseal region. Various studies on the function of PT cells have assumed that these cells are involved in liquid traffic between the hypothalamo-hypophyseal portal vessels and the cerebrospinal fluid toward the subarachnoidal space and the third ventricle (15, 16). Because the function of guanylin is well documented with respect to regulation of electrolyte/water homeostasis, the unique localization of guanylin in the PT-specific cells implicates a pivotal regulatory role of guanylin in these cells. Moreover, the PT is one of the zones where the melatonin receptors reach their highest concentration (26). Hence, PT is currently considered the most important mediator between pineal and hypophyseal PD functions by transferring the pineal inputs generated by the photoperiod to the PD secretory activity overall of gonadotrophic hormones and prolactin (see ref. 25 for a review). Because guanylin is expressed both in the rostral PT cells and in the PD gonadotrophs, guanylin is a potential candidate of regulatory factors involved in this particular intermediary function.
Acknowledgments
We warmly thank Professor Franco Marchiolli for his skillful help in computer-aided composition of figures. This study was supported by grants from the Italian Ministero dell' Università e della Ricerca Scientifica e Tecnologica (to L.D. and T.G.R.), from the Deutsche Forschungsgemeinschaft (Ce32/1-2), and from the Hungarian Országos Tudományos Kutatási Alap (Grant 029172 to T.W.).
Abbreviations
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- PBST
PBS/BSA containing 0.3% Triton X-100
- PD
pars distalis of pituitary gland
- PT
pars tuberalis of pituitary gland
- RT-PCR
reverse transcriptase–PCR
- h-β-LH
anti-human luteinizing hormone
- h-β-TSH
anti-human thyroid stimulating hormone
- anti-GFAP
anti-glial fibrillary acid protein
Footnotes
This paper was approved for publication by Vittorio Erspamer before his death (October 26, 1999) but was not received in the PNAS office until November 29, 1999.
References
- 1.Currie M G, Fok K F, Kato J, Moore R J, Hamra F K, Duffin K L, Smith C E. Proc Natl Acad Sci USA. 1992;89:947–961. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabers D L. Cell. 1992;71:1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- 3.De Sauvage F, Keshav S, Kuang W J. Proc Natl Acad Sci USA. 1992;89:9089–9093. doi: 10.1073/pnas.89.19.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamra F K, Forte L R, Eber S L, Pidhorodeckyi N V, Krause W J, Freeman R H, Chin D T, Tompkin J A, Fok K F, Smith C E, et al. Proc Natl Acad Sci USA. 1993;90:10464–10468. doi: 10.1073/pnas.90.22.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forte L R, Eber S L, Fan X, London R M, Wang Y, Rowland L M, Chin D T, Freeman R H, Krause W J. Endocrinology. 1999;140:1800–1806. doi: 10.1210/endo.140.4.6630. [DOI] [PubMed] [Google Scholar]
- 6.Forte L R, Currie M G. FASEB J. 1995;9:643–650. doi: 10.1096/fasebj.9.8.7768356. [DOI] [PubMed] [Google Scholar]
- 7.Lewis L G, Witte D, Laney D W, Currie M, Cohen M. Biochem Biophys Res Commun. 1993;196:553–560. doi: 10.1006/bbrc.1993.2285. [DOI] [PubMed] [Google Scholar]
- 8.Cetin Y, Kuhn M, Kulaksiz H, Adermann K, Bargsten G, Grube D, Forssmann W-G. Proc Natl Acad Sci USA. 1994;91:2935–2939. doi: 10.1073/pnas.91.8.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Taylor-Blake B, Light A R, Goy M F. Gastroenterology. 1995;109:1863–1875. doi: 10.1016/0016-5085(95)90753-x. [DOI] [PubMed] [Google Scholar]
- 10.Hamra F K, Krause W J, Eber S L, Freeman R H, Smith C E, Currie M G, Forte L R. Am J Physiol. 1996;270:G708–G716. doi: 10.1152/ajpgi.1996.270.4.G708. [DOI] [PubMed] [Google Scholar]
- 11.Miyazato M, Nakazato M, Matsukura S, Kangawa K, Matsuo H. FEBS Lett. 1996;398:170–174. doi: 10.1016/s0014-5793(96)01235-5. [DOI] [PubMed] [Google Scholar]
- 12.London R M, Krause W J, Fan X, Eber S L, Forte L R. Am J Physiol. 1997;273:G93–G105. doi: 10.1152/ajpgi.1997.273.1.G93. [DOI] [PubMed] [Google Scholar]
- 13.Cetin Y, Kulaksiz H, Redecker P, Bargsten G, Adermann K, Grube D, Forssmann W-G. Proc Natl Acad Sci USA. 1995;92:5925–5929. doi: 10.1073/pnas.92.13.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn M, Raida M, Adermann K, Schulz-Knappe P, Gerzer R, Heim J-M, Forrssmann W-G. FEBS Lett. 1993;318:205–209. doi: 10.1016/0014-5793(93)80022-m. [DOI] [PubMed] [Google Scholar]
- 15.Aguado L I, Schoebitz K, Rodriguez E M. Cell Tissue Res. 1981;218:345–354. doi: 10.1007/BF00210349. [DOI] [PubMed] [Google Scholar]
- 16.Wittkowski W H, Schulze-Bonhage A H, Bockers T M. Acta Endocrinol. 1992;126:285–290. doi: 10.1530/acta.0.1260285. [DOI] [PubMed] [Google Scholar]
- 17.Fourney R M, Miyakoshi J, Day R S, Paterson M C. Focus. 1988;10:5–7. [Google Scholar]
- 18.Lee C C, Caskey C T. In: PCR Protocols: A Guide to Methods and Applications. Innis M A, Gelfand D H, Sninsky J J, White T J, editors. San Diego: Academic; 1990. pp. 3–12. [Google Scholar]
- 19.Corpet F. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Innis M A, Gelfand D H. In: PCR Protocols: A Guide to Methods and Applications. Innis M A, Gelfand D H, Sninsky J J, White T J, editors. San Diego: Academic; 1990. pp. 46–53. [Google Scholar]
- 21.Scagger H, von Jagow G. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 22.Li C Y, Ziesmer S C, Lazcano-Villareal O. J Histochem Cytochem. 1987;35:1457–1460. doi: 10.1177/35.12.2824601. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald K T. Gen Comp Endocrinol. 1979;37:383–399. doi: 10.1016/0016-6480(79)90012-1. [DOI] [PubMed] [Google Scholar]
- 24.Stoeckel M-E, Porte A. In: Ultrastructure of Endocrine Cells and Tissue. Motta P M, editor. The Haguel, Netherlands: Martinus Nijhoff; 1984. pp. 29–38. [Google Scholar]
- 25.Wittkowski W, Bockmann J, Kreutz M R, Böckers T M. Int Rev Cytol. 1999;185:157–194. doi: 10.1016/s0074-7696(08)60151-5. [DOI] [PubMed] [Google Scholar]
- 26.Williams L M, Morgan P J. J Endocrinol. 1988;119:R1–R3. doi: 10.1677/joe.0.119r001. [DOI] [PubMed] [Google Scholar]