Abstract
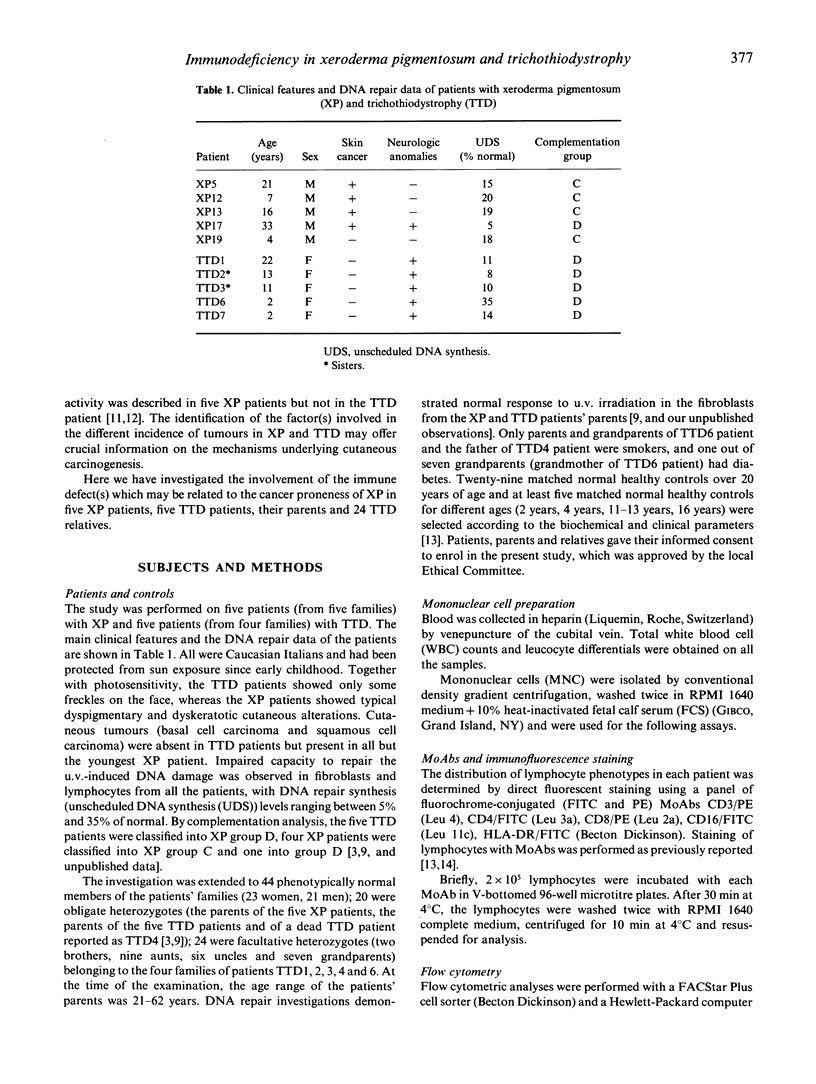
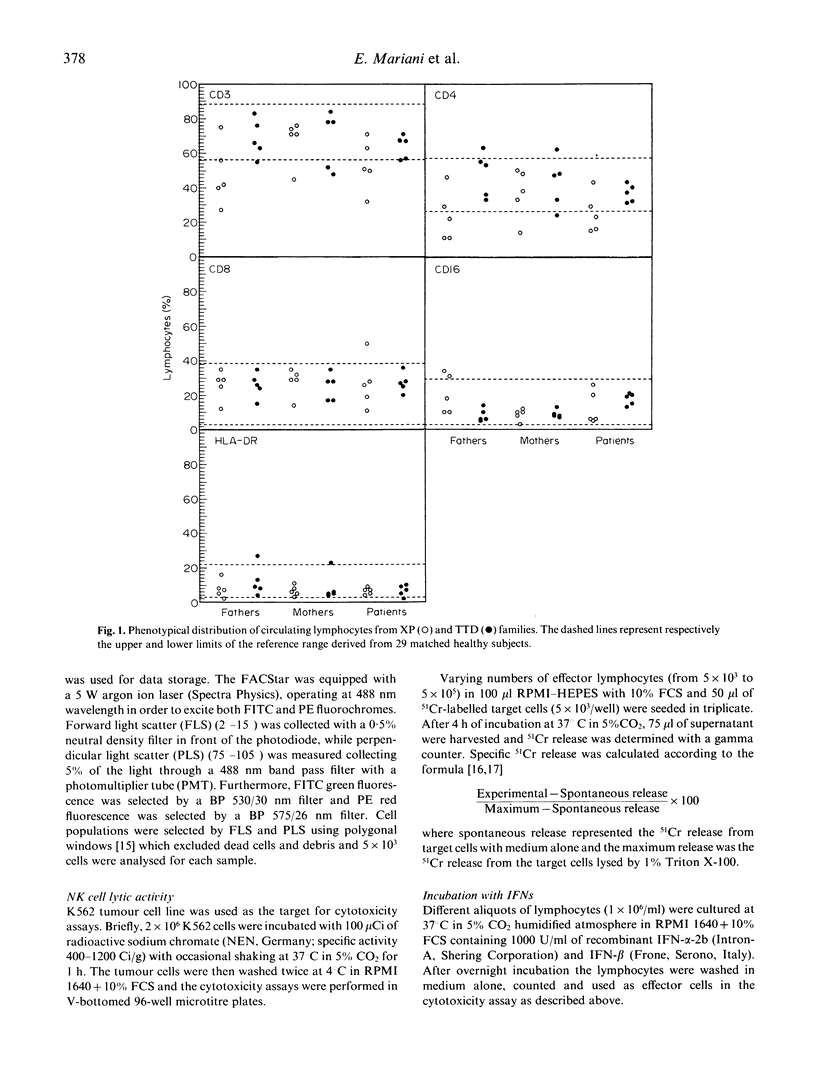
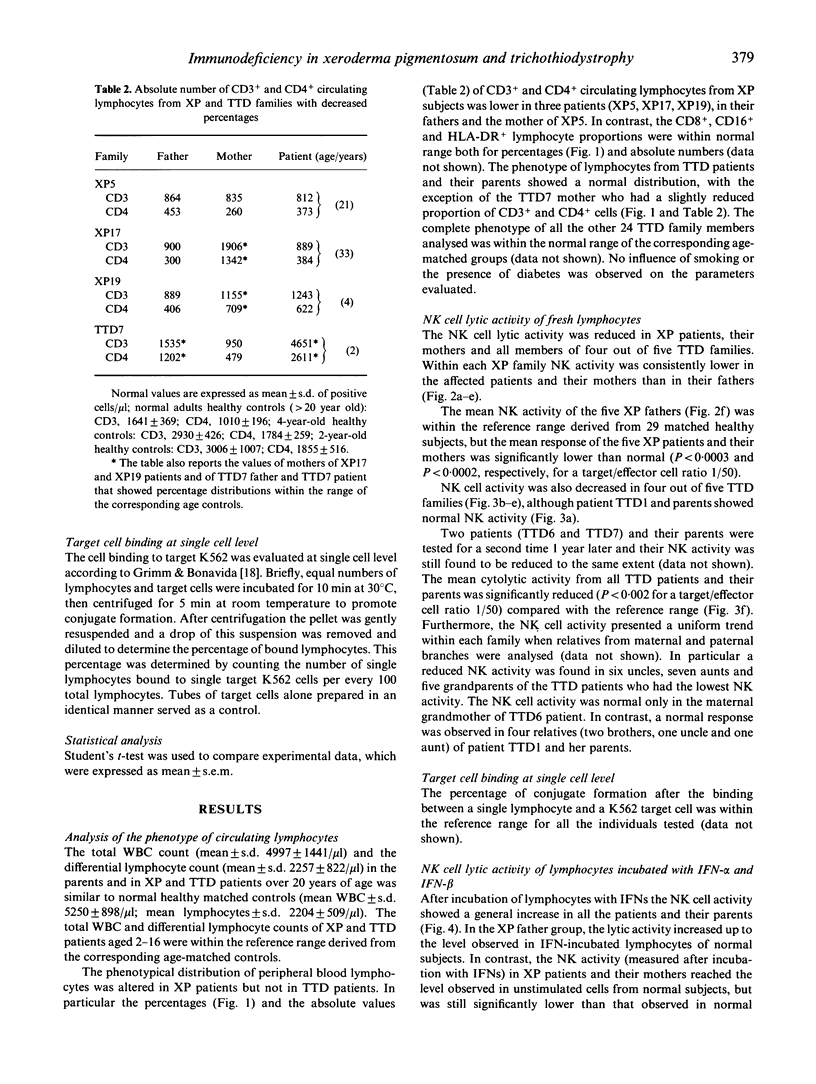
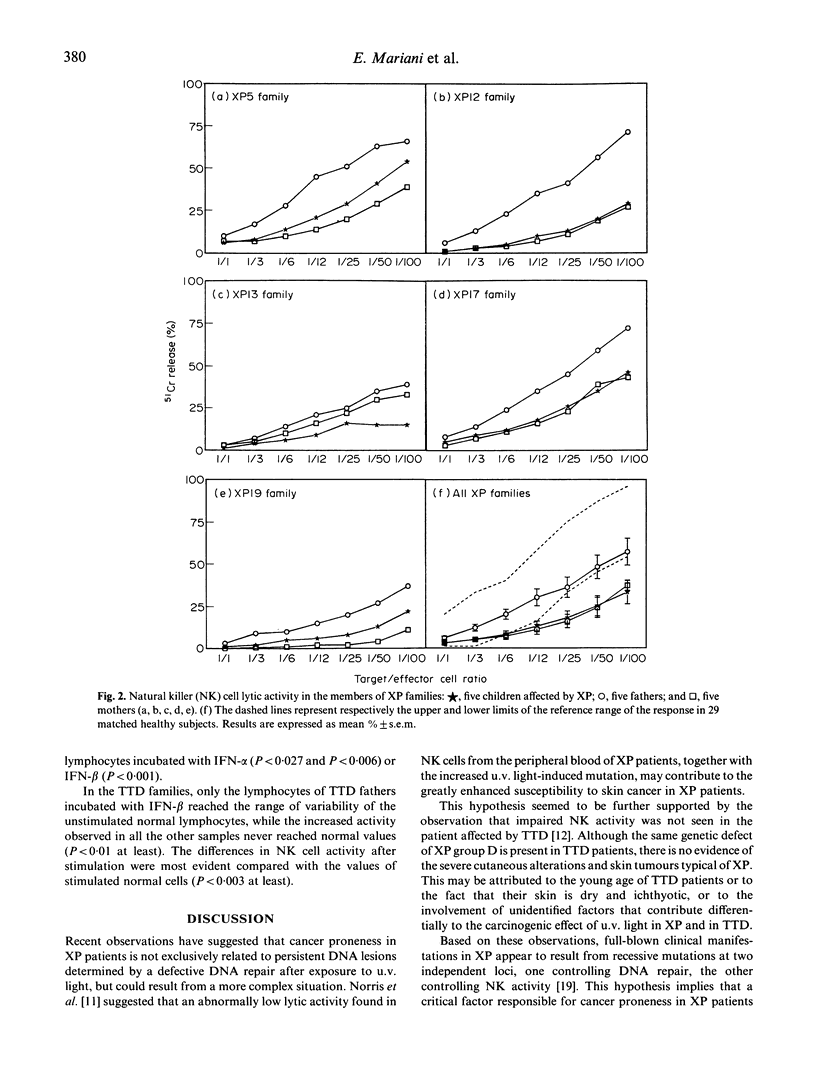
Xeroderma pigmentosum (XP) is a rare autosomal recessive disease characterized by photosensitivity, a high incidence of cancer in sun-exposed portions of the skin and a reduced capacity to repair the u.v.-induced DNA damage. One of the XP mutations (XP-D) has also been identified in patients affected by trichothiodystrophy (TTD), a rare autosomal recessive disease characterized by brittle hair, mental and physical retardation, peculiar face and ichthyosis. However, in these patients there is no evidence of increased skin tumour incidence. Since an impairment of cell-mediated immunity has been proposed as a co-factor in the cancer proneness of XP patients, we investigated the involvement of immune defect(s) in five XP patients, five TTD patients, their parents, and 24 TTD relatives. We evaluated the phenotype of circulating lymphocytes, natural killer (NK) cell lytic activity, target cell binding of NK cells at single cell level and the effect of interferons (IFN) alpha and beta on NK cell activity. The relative proportion of CD3+ and CD4+ circulating lymphocytes was reduced in XP but not in TTD patients. NK cell lytic activity was decreased in XP patients and their mothers, but their fathers showed normal lytic activity. NK activity varied among TTD families: four out of five patients and their relatives presented low NK cell activity, and one family was normal. In TTD family members, NK activity increased after incubation with IFN-alpha or IFN-beta, but never reached normal values. In contrast, in XP patients and their mothers, the defect was almost completely corrected after in vitro incubation with IFN-alpha or IFN-beta. Our study indicates impaired NK lytic activity in the majority of TTD and XP patients and that this defect is present also in members of their families. In addition, XP patients present a low number of circulating T cells. These multiple abnormalities, together with DNA repair defects, could be related to the increased cancer risk in XP patients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Facchini A., Mariani E., Mariani A. R., Papa S., Vitale M., Manzoli F. A. Increased number of circulating Leu 11+ (CD 16) large granular lymphocytes and decreased NK activity during human ageing. Clin Exp Immunol. 1987 May;68(2):340–347. [PMC free article] [PubMed] [Google Scholar]
- Grimm E., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. I. Estimation of cytotoxic T lymphocyte frequency and relative lytic efficiency. J Immunol. 1979 Dec;123(6):2861–2869. [PubMed] [Google Scholar]
- Hoeijmakers J. H., Bootsma D. Molecular genetics of eukaryotic DNA excision repair. Cancer Cells. 1990 Oct;2(10):311–320. [PubMed] [Google Scholar]
- Itin P. H., Pittelkow M. R. Trichothiodystrophy: review of sulfur-deficient brittle hair syndromes and association with the ectodermal dysplasias. J Am Acad Dermatol. 1990 May;22(5 Pt 1):705–717. doi: 10.1016/0190-9622(90)70096-z. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Arlett C. F., Broughton B. C., Harcourt S. A., Steingrimsdottir H., Stefanini M., Malcolm A., Taylor R., Natarajan A. T., Green S. Trichothiodystrophy, a human DNA repair disorder with heterogeneity in the cellular response to ultraviolet light. Cancer Res. 1988 Nov 1;48(21):6090–6096. [PubMed] [Google Scholar]
- Lehmann A. R., Norris P. G. DNA repair and cancer: speculations based on studies with xeroderma pigmentosum, Cockayne's syndrome and trichothiodystrophy. Carcinogenesis. 1989 Aug;10(8):1353–1356. doi: 10.1093/carcin/10.8.1353. [DOI] [PubMed] [Google Scholar]
- Mariani E., Facchini A., Miglio F., Stefanini G. F., Mazzetti M., Leupers T., Gasbarrini G., Labò G., Astaldi A. Analysis with OKT monoclonal antibodies of T-lymphocyte subsets present in blood and liver of patients with chronic active hepatitis. Liver. 1984 Feb;4(1):22–28. doi: 10.1111/j.1600-0676.1984.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Mariani E., Roda P., Mariani A. R., Vitale M., Degrassi A., Papa S., Facchini A. Age-associated changes in CD8+ and CD16+ cell reactivity: clonal analysis. Clin Exp Immunol. 1990 Sep;81(3):479–484. doi: 10.1111/j.1365-2249.1990.tb05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris P. G., Limb G. A., Hamblin A. S., Hawk J. L. Impairment of natural-killer-cell activity in xeroderma pigmentosum. N Engl J Med. 1988 Dec 22;319(25):1668–1669. doi: 10.1056/NEJM198812223192512. [DOI] [PubMed] [Google Scholar]
- Norris P. G., Limb G. A., Hamblin A. S., Lehmann A. R., Arlett C. F., Cole J., Waugh A. P., Hawk J. L. Immune function, mutant frequency, and cancer risk in the DNA repair defective genodermatoses xeroderma pigmentosum, Cockayne's syndrome, and trichothiodystrophy. J Invest Dermatol. 1990 Jan;94(1):94–100. doi: 10.1111/1523-1747.ep12873952. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Bonnard G. D., Herberman R. B. Cytotoxic reactivity of human lymphocytes cultured in vitro. J Immunol. 1977 Oct;119(4):1351–1357. [PubMed] [Google Scholar]
- Stefanini M., Lagomarsini P., Arlett C. F., Marinoni S., Borrone C., Crovato F., Trevisan G., Cordone G., Nuzzo F. Xeroderma pigmentosum (complementation group D) mutation is present in patients affected by trichothiodystrophy with photosensitivity. Hum Genet. 1986 Oct;74(2):107–112. doi: 10.1007/BF00282072. [DOI] [PubMed] [Google Scholar]
- Stefanini M., Lagomarsini P., Giorgi R., Nuzzo F. Complementation studies in cells from patients affected by trichothiodystrophy with normal or enhanced UV photosensitivity. Mutat Res. 1987 Jun;191(2):117–119. doi: 10.1016/0165-7992(87)90139-4. [DOI] [PubMed] [Google Scholar]
- Vitale M., Papa S., Mariani A. R., Facchini A., Rizzoli R., Manzoli F. A. Use of poligonal windows for physical discrimination among mononuclear subpopulations in flow cytometry. J Immunol Methods. 1987 Jan 26;96(1):63–68. doi: 10.1016/0022-1759(87)90368-1. [DOI] [PubMed] [Google Scholar]
- Wysenbeek A. J., Weiss H., Duczyminer-Kahana M., Grunwald M. H., Pick A. I. Immunologic alterations in xeroderma pigmentosum patients. Cancer. 1986 Jul 15;58(2):219–221. doi: 10.1002/1097-0142(19860715)58:2<219::aid-cncr2820580203>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]


