Abstract
Using an experimental model of autoimmune uveoretinitis, we have examined the relationship of T cell infiltration in the retina to blood-retinal barrier (BRB) breakdown. Sensitive quantitative in vivo techniques were used to examine BRB permeability to sucrose, a low mol. wt non-transported solute. Electron microscopy was also used to localize extravasated horseradish peroxidase, a macromolecular visual tracer, from the retinal vasculature and to identify the route by which any leakage was occurring. No increase in BRB permeability was found prior to lymphocytic infiltration. By day 10 of the disease inflammatory cells could be seen within the structurally intact retina, which was shortly followed by an increase in the permeability of the BRB to sucrose. Only later in the disease process, when damage to the photoreceptor layer became apparent, did extravasation of the macromolecule HRP occur. At no stage of the disease process was there any detectable damage to inter-endothelial tight junctions. The size-dependancy of tracer extravasation in the initial stages of the disease is indicative of a paracellular route being responsible for the increase in BRB permeability. In later stages of the disease some evidence of horseradish peroxidase filled 'vesicle-like' profiles was observed. We suggest that the devastating complication of BRB breakdown in ocular inflammation is a direct consequence of lymphocytic infiltration.
Full text
PDF

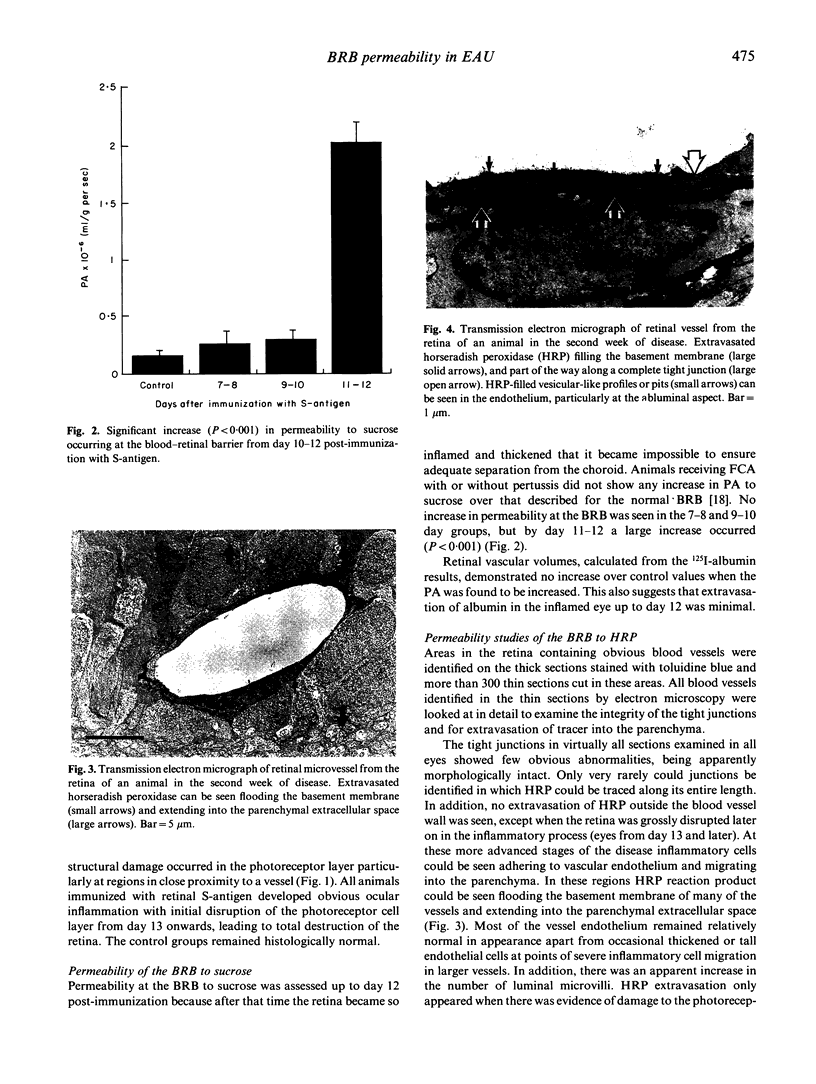


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Mahdawi S., Forrester J. V., Lee W. R. A simplified method for the isolation of highly purified bovine retinal S antigen. J Neuroimmunol. 1987 Feb;14(1):99–108. doi: 10.1016/0165-5728(87)90104-4. [DOI] [PubMed] [Google Scholar]
- Broadwell R. D. Transcytosis of macromolecules through the blood-brain barrier: a cell biological perspective and critical appraisal. Acta Neuropathol. 1989;79(2):117–128. doi: 10.1007/BF00294368. [DOI] [PubMed] [Google Scholar]
- Brosnan C. F., Claudio L., Tansey F. A., Martiney J. Mechanisms of autoimmune neuropathies. Ann Neurol. 1990;27 (Suppl):S75–S79. doi: 10.1002/ana.410270719. [DOI] [PubMed] [Google Scholar]
- Brosnan C. F., Goldmuntz E. A., Cammer W., Factor S. M., Bloom B. R., Norton W. T. Prazosin, an alpha 1-adrenergic receptor antagonist, suppresses experimental autoimmune encephalomyelitis in the Lewis rat. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5915–5919. doi: 10.1073/pnas.82.17.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi R. R., Roberge F. G., McAllister C. G., el-Saied M., Kuwabara T., Gery I., Hanna E., Nussenblatt R. B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [PubMed] [Google Scholar]
- Charteris D. G., Lee W. R. Multifocal posterior uveitis: clinical and pathological findings. Br J Ophthalmol. 1990 Nov;74(11):688–693. doi: 10.1136/bjo.74.11.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charteris D. G., Lightman S. L. Interferon-gamma (IFN-gamma) production in vivo in experimental autoimmune uveoretinitis. Immunology. 1992 Mar;75(3):463–467. [PMC free article] [PubMed] [Google Scholar]
- Claudio L., Kress Y., Factor J., Brosnan C. F. Mechanisms of edema formation in experimental autoimmune encephalomyelitis. The contribution of inflammatory cells. Am J Pathol. 1990 Nov;137(5):1033–1045. [PMC free article] [PubMed] [Google Scholar]
- Claudio L., Kress Y., Norton W. T., Brosnan C. F. Increased vesicular transport and decreased mitochondrial content in blood-brain barrier endothelial cells during experimental autoimmune encephalomyelitis. Am J Pathol. 1989 Dec;135(6):1157–1168. [PMC free article] [PubMed] [Google Scholar]
- Daniel P. M., Lam D. K., Pratt O. E. Relation between the increase in the diffusional permeability of the blood-central nervous system barrier and other changes during the development of experimental allergic encephalomyelitis in the Lewis rat. J Neurol Sci. 1983 Aug-Sep;60(3):367–376. doi: 10.1016/0022-510x(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Dua H. S., McKinnon A., McMenamin P. G., Forrester J. V. Ultrastructural pathology of the 'barrier sites' in experimental autoimmune uveitis and experimental autoimmune pinealitis. Br J Ophthalmol. 1991 Jul;75(7):391–397. doi: 10.1136/bjo.75.7.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner E. Role of vesicular transport in breakdown of the blood-retinal barrier. Lab Invest. 1987 May;56(5):457–460. [PubMed] [Google Scholar]
- Greenwood J. Mechanisms of blood-brain barrier breakdown. Neuroradiology. 1991;33(2):95–100. doi: 10.1007/BF00588242. [DOI] [PubMed] [Google Scholar]
- Hawkins C. P., Munro P. M., MacKenzie F., Kesselring J., Tofts P. S., du Boulay E. P., Landon D. N., McDonald W. I. Duration and selectivity of blood-brain barrier breakdown in chronic relapsing experimental allergic encephalomyelitis studied by gadolinium-DTPA and protein markers. Brain. 1990 Apr;113(Pt 2):365–378. doi: 10.1093/brain/113.2.365. [DOI] [PubMed] [Google Scholar]
- Hickey W. F. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991 Jan;1(2):97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Juhler M. Pathophysiological aspects of acute experimental allergic encephalomyelitis. Acta Neurol Scand Suppl. 1988;119:1–21. doi: 10.1111/j.1600-0404.1988.tb08016.x. [DOI] [PubMed] [Google Scholar]
- Kato S., Nakamura H. Ultrastructural and ultracytochemical studies on the blood-brain barrier in chronic relapsing experimental allergic encephalomyelitis. Acta Neuropathol. 1989;77(5):455–464. doi: 10.1007/BF00687246. [DOI] [PubMed] [Google Scholar]
- Kerlero de Rosbo N., Bernard C. C., Simmons R. D., Carnegie P. R. Concomitant detection of changes in myelin basic protein and permeability of blood-spinal cord barrier in acute experimental autoimmune encephalomyelitis by electroimmunoblotting. J Neuroimmunol. 1985 Oct;9(6):349–361. doi: 10.1016/s0165-5728(85)80035-7. [DOI] [PubMed] [Google Scholar]
- Lightman S. L., Palestine A. G., Rapoport S. I., Rechthand E. Quantitative assessment of the permeability of the rat blood-retinal barrier to small water-soluble non-electrolytes. J Physiol. 1987 Aug;389:483–490. doi: 10.1113/jphysiol.1987.sp016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman S., Chan C. C. Immune mechanisms in choroido-retinal inflammation in man. Eye (Lond) 1990;4(Pt 2):345–353. doi: 10.1038/eye.1990.47. [DOI] [PubMed] [Google Scholar]
- Lightman S., Pinter G., Yuen L., Bradbury M. Permeability changes at blood-retinal barrier in diabetes and effect of aldose reductase inhibition. Am J Physiol. 1990 Sep;259(3 Pt 2):R601–R605. doi: 10.1152/ajpregu.1990.259.3.R601. [DOI] [PubMed] [Google Scholar]
- Male D., Pyrce G., Hughes C., Lantos P. Lymphocyte migration into brain modelled in vitro: control by lymphocyte activation, cytokines, and antigen. Cell Immunol. 1990 Apr 15;127(1):1–11. doi: 10.1016/0008-8749(90)90109-5. [DOI] [PubMed] [Google Scholar]
- Merchant R. E., Ellison M. D., Young H. F. Immunotherapy for malignant glioma using human recombinant interleukin-2 and activated autologous lymphocytes. A review of pre-clinical and clinical investigations. J Neurooncol. 1990 Apr;8(2):173–188. doi: 10.1007/BF00177842. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Cohen I. R., Fuks Z., Vlodavsky I. Activated T lymphocytes produce a matrix-degrading heparan sulphate endoglycosidase. Nature. 1984 Jul 19;310(5974):241–244. doi: 10.1038/310241a0. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Cannella B., Duijvestijn A. M., Cross A. H. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab Invest. 1990 Oct;63(4):476–489. [PubMed] [Google Scholar]
- Robinson P. J., Rapoport S. I. Size selectivity of blood-brain barrier permeability at various times after osmotic opening. Am J Physiol. 1987 Sep;253(3 Pt 2):R459–R466. doi: 10.1152/ajpregu.1987.253.3.R459. [DOI] [PubMed] [Google Scholar]
- Savion N., Vlodavsky I., Fuks Z. Interaction of T lymphocytes and macrophages with cultured vascular endothelial cells: attachment, invasion, and subsequent degradation of the subendothelial extracellular matrix. J Cell Physiol. 1984 Feb;118(2):169–178. doi: 10.1002/jcp.1041180209. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Mason D. W. The mechanism of inhibition of experimental allergic encephalomyelitis in the rat by monoclonal antibody against CD4. J Neuroimmunol. 1986 Dec;13(2):217–232. doi: 10.1016/0165-5728(86)90066-4. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stanford M. R., Atkinson E., Kasp E., Dumonde D. C. Modulation of experimental retinal vasculitis using dexamethasone, cyclosporin A, and prazosin. Eye (Lond) 1987;1(Pt 5):626–631. doi: 10.1038/eye.1987.97. [DOI] [PubMed] [Google Scholar]
- Stohl W., Kaplan M. S., Gonatas N. K. A quantitative assay for experimental allergic encephalomyelitis in the rat based on permeability of spinal cords to 125I-human gamma-globulin. J Immunol. 1979 Mar;122(3):920–925. [PubMed] [Google Scholar]
- Wekerle H., Engelhardt B., Risau W., Meyermann R. Interaction of T lymphocytes with cerebral endothelial cells in vitro. Brain Pathol. 1991 Jan;1(2):107–114. doi: 10.1111/j.1750-3639.1991.tb00647.x. [DOI] [PubMed] [Google Scholar]
- de Kozak Y., Sakai J., Thillaye B., Faure J. P. S antigen-induced experimental autoimmune uveo-retinitis in rats. Curr Eye Res. 1981;1(6):327–337. doi: 10.3109/02713688108998359. [DOI] [PubMed] [Google Scholar]





