Abstract
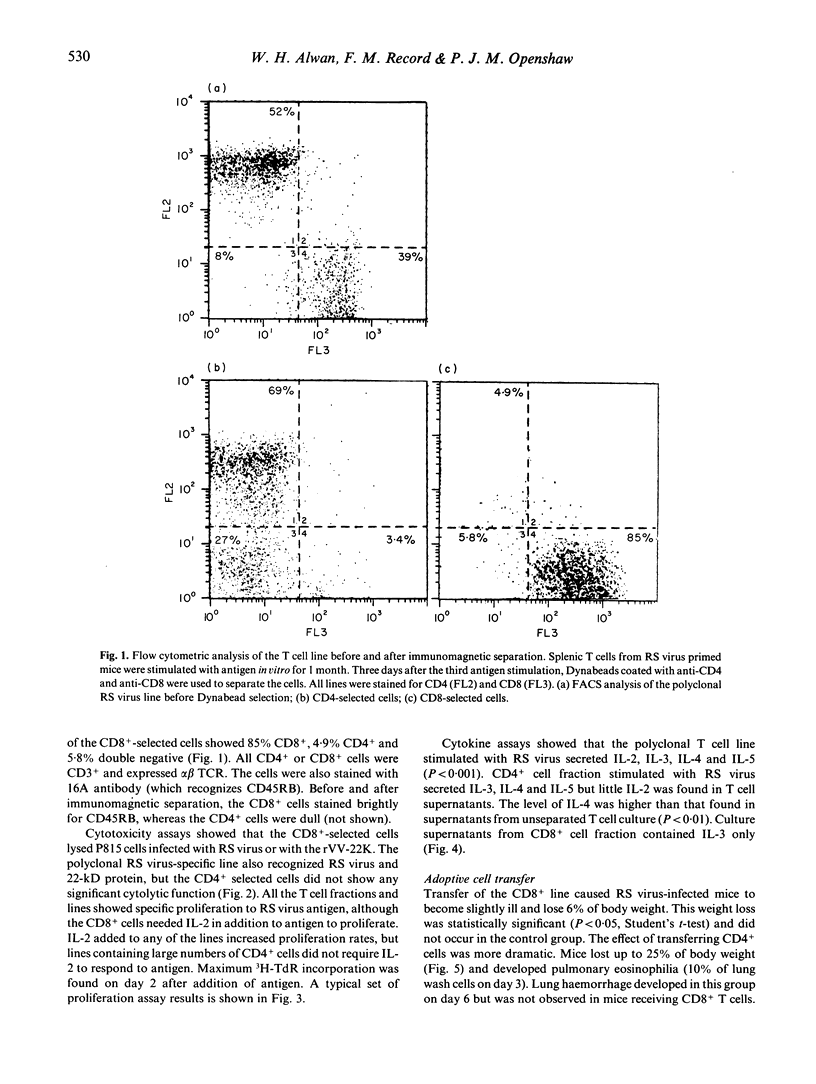
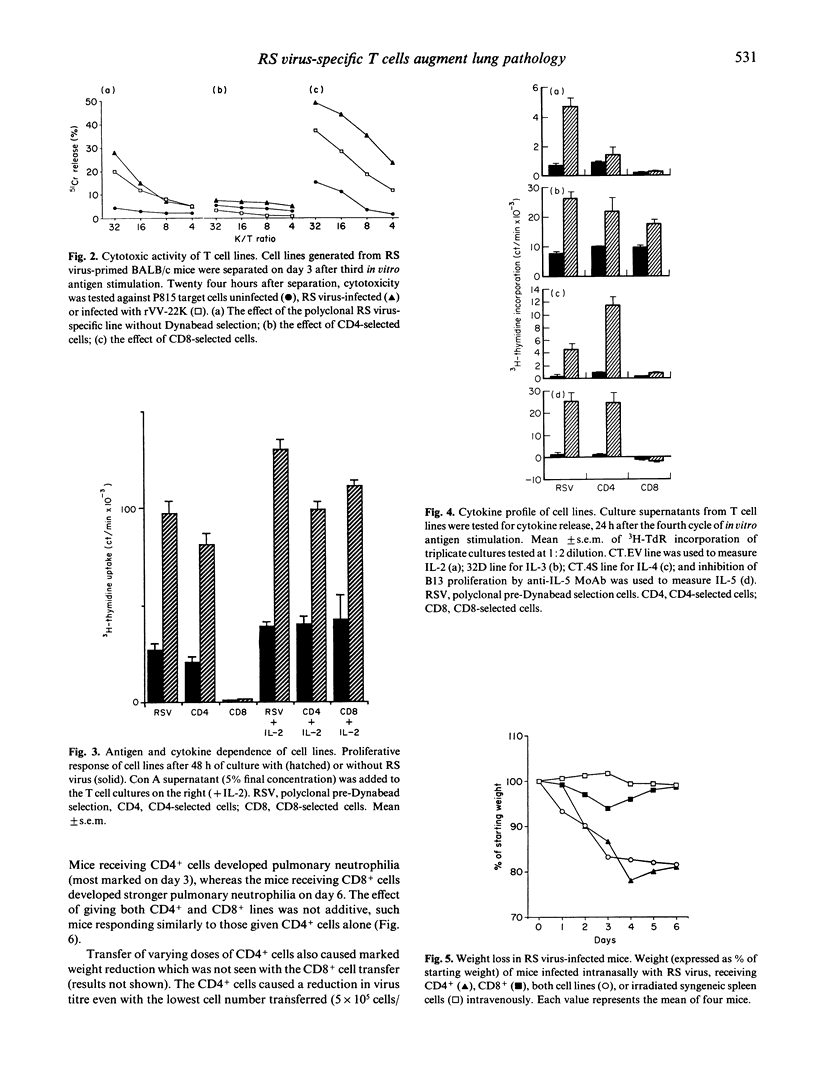
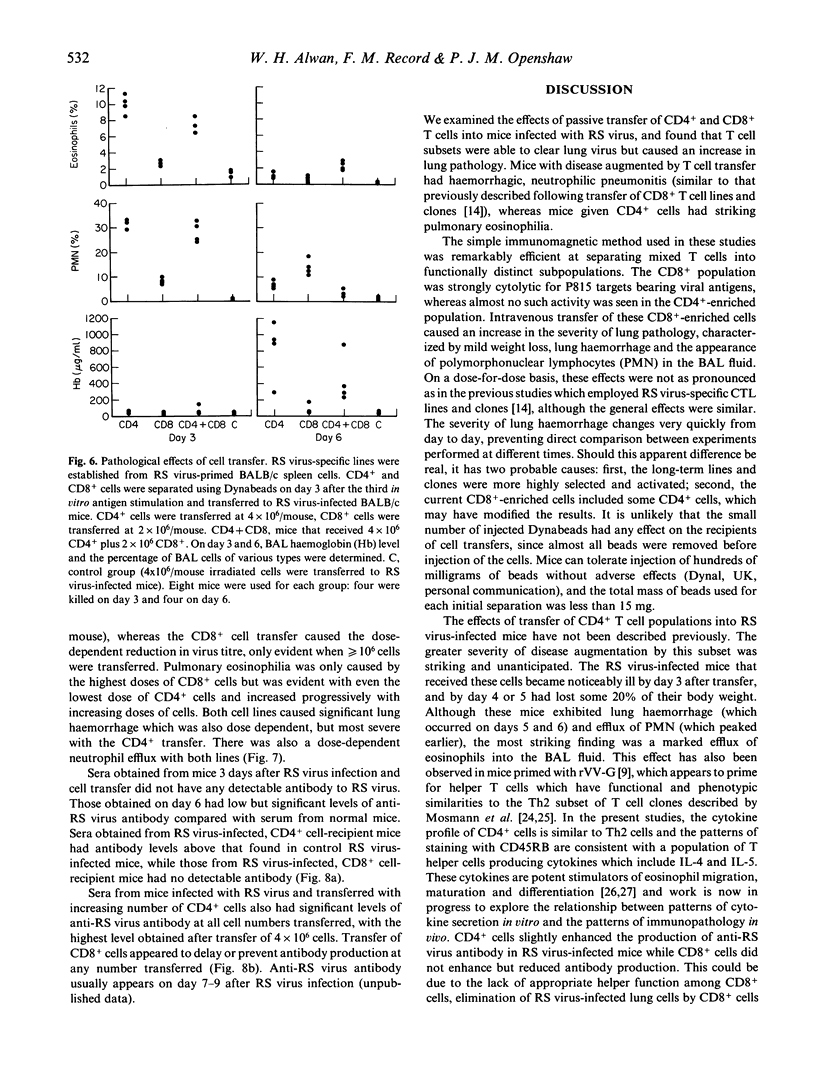
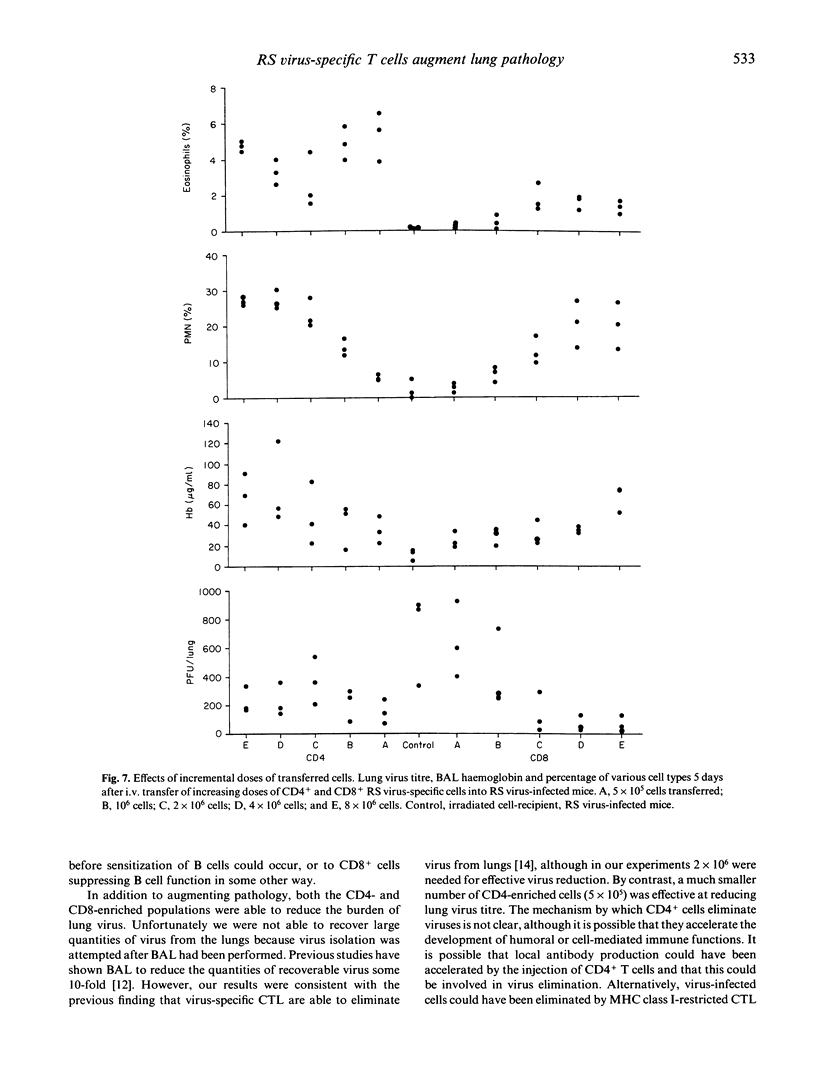
Respiratory syncytial (RS) virus-specific T cell lines were derived from the spleens of BALB/c mice primed by intranasal infection with RS virus. The lines were expanded by repeated antigenic stimulation in vitro, and separated into CD4+ and CD8+ T cell-enriched fractions by immunomagnetic adhesion. The effects of passive transfer of these fractions into RS virus infected mice were observed. The most severe immunopathological changes were seen in mice receiving CD4+ cells. Transfer of CD4+, CD8+ or both cell fractions caused RS virus-infected mice to become ill and lose weight. Both cell lines caused an increase in the severity of lung pathology (as monitored by bronchoalveolar lavage) with the appearance of lung haemorrhage and polymorphonuclear cell efflux. In addition, recipients of CD4+ cells developed striking pulmonary eosinophilia. In CD4+ cell recipients, 5 x 10(5) cells were sufficient to decrease lung virus titre, whereas 2 x 10(6) CD8+ cells were needed to produce a similar effect. The unseparated T cell line and the CD4+ cell fraction secreted significant amounts of IL-3, IL-4 and IL-5 (P less than 0.001). High levels of IL-2 were produced only by the unseparated T cell line. The CD8+ cell fraction secreted IL-3 only. The results show that, cell-for-cell, CD4+ cells are more anti-viral and more immunopathogenic than CD8+ cells in RS virus infected mice. Such effects may have contributed to the augmented disease seen in some infants vaccinated against RS virus.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Norden J., Saunders D., Toms G. L., Scott R. Analysis of the local and systemic immune responses induced in BALB/c mice by experimental respiratory syncytial virus infection. J Gen Virol. 1990 Jul;71(Pt 7):1561–1570. doi: 10.1099/0022-1317-71-7-1561. [DOI] [PubMed] [Google Scholar]
- Anderson J. J., Serin M., Harrop J., Amin S., Toms G. L., Scott R. Natural killer cell response to respiratory syncytial virus in the Balb/c mouse model. Adv Exp Med Biol. 1989;257:211–220. doi: 10.1007/978-1-4684-5712-4_20. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Van Voris L. P., Mufson M. A. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. J Infect Dis. 1982 Mar;145(3):311–319. doi: 10.1093/infdis/145.3.311. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Schröppel K., Lohoff M., Röllinghoff M., Solbach W. Immunization of susceptible hosts with a soluble antigen fraction from Leishmania major leads to aggravation of murine leishmaniasis mediated by CD4+ T cells. Eur J Immunol. 1990 Dec;20(12):2533–2540. doi: 10.1002/eji.1830201202. [DOI] [PubMed] [Google Scholar]
- Cannon M. J. Microplaque immunoperoxidase detection of infectious respiratory syncytial virus in the lungs of infected mice. J Virol Methods. 1987 Jul;16(4):293–301. doi: 10.1016/0166-0934(87)90014-0. [DOI] [PubMed] [Google Scholar]
- Cannon M. J., Openshaw P. J., Askonas B. A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988 Sep 1;168(3):1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M. J., Stott E. J., Taylor G., Askonas B. A. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology. 1987 Sep;62(1):133–138. [PMC free article] [PubMed] [Google Scholar]
- Castleman W. L., Sorkness R. L., Lemanske R. F., Jr, McAllister P. K. Viral bronchiolitis during early life induces increased numbers of bronchiolar mast cells and airway hyperresponsiveness. Am J Pathol. 1990 Oct;137(4):821–831. [PMC free article] [PubMed] [Google Scholar]
- Cherrie A. H., Anderson K., Wertz G. W., Openshaw P. J. Human cytotoxic T cells stimulated by antigen on dendritic cells recognize the N, SH, F, M, 22K, and 1b proteins of respiratory syncytial virus. J Virol. 1992 Apr;66(4):2102–2110. doi: 10.1128/jvi.66.4.2102-2110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J., Magoffin R. L., Shearer L. A., Schieble J. H., Lennette E. H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969 Apr;89(4):449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- De Monchy J. G., Kauffman H. F., Venge P., Koëter G. H., Jansen H. M., Sluiter H. J., De Vries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis. 1985 Mar;131(3):373–376. doi: 10.1164/arrd.1985.131.3.373. [DOI] [PubMed] [Google Scholar]
- Dementia--the quiet epidemic. Br Med J. 1978 Jan 7;1(6104):1–2. [PMC free article] [PubMed] [Google Scholar]
- Dent L. A., Strath M., Mellor A. L., Sanderson C. J. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990 Nov 1;172(5):1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigas E., Gleich G. J. The eosinophil and the pathophysiology of asthma. J Allergy Clin Immunol. 1986 Apr;77(4):527–537. doi: 10.1016/0091-6749(86)90341-6. [DOI] [PubMed] [Google Scholar]
- Graham B. S., Perkins M. D., Wright P. F., Karzon D. T. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988 Oct;26(2):153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M. D., Winters S. T., Olee T., Powell H. C., Carlo D. J., Brostoff S. W. Vaccination against experimental allergic encephalomyelitis with T cell receptor peptides. Science. 1989 Nov 3;246(4930):668–670. doi: 10.1126/science.2814489. [DOI] [PubMed] [Google Scholar]
- Hu-Li J., Ohara J., Watson C., Tsang W., Paul W. E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S). J Immunol. 1989 Feb 1;142(3):800–807. [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Karpus W. J., Swanborg R. H. CD4+ suppressor cells differentially affect the production of IFN-gamma by effector cells of experimental autoimmune encephalomyelitis. J Immunol. 1989 Dec 1;143(11):3492–3497. [PubMed] [Google Scholar]
- Kast W. M., Bronkhorst A. M., de Waal L. P., Melief C. J. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC-restricted and MHC-regulated; a model for MHC-disease associations. J Exp Med. 1986 Sep 1;164(3):723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattan M., Keens T. G., Lapierre J. G., Levison H., Bryan A. C., Reilly B. J. Pulmonary function abnormalities in symptom-free children after bronchiolitis. Pediatrics. 1977 May;59(5):683–688. [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Openshaw P. J., Anderson K., Wertz G. W., Askonas B. A. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol. 1990 Apr;64(4):1683–1689. doi: 10.1128/jvi.64.4.1683-1689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw P. J., Clarke S. L., Record F. M. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992 Apr;4(4):493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- Openshaw P. J. Flow cytometric analysis of pulmonary lymphocytes from mice infected with respiratory syncytial virus. Clin Exp Immunol. 1989 Feb;75(2):324–328. [PMC free article] [PubMed] [Google Scholar]
- Openshaw P. J., Pemberton R. M., Ball L. A., Wertz G. W., Askonas B. A. Helper T cell recognition of respiratory syncytial virus in mice. J Gen Virol. 1988 Feb;69(Pt 2):305–312. doi: 10.1099/0022-1317-69-2-305. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Hemming V. G., Horswood R. L., Baron P. A., Chanock R. M. Effectiveness of topically administered neutralizing antibodies in experimental immunotherapy of respiratory syncytial virus infection in cotton rats. J Virol. 1987 Jun;61(6):1851–1854. doi: 10.1128/jvi.61.6.1851-1854.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Hemming V. G., Murphy B. R., Walsh E. E., Horswood R. L., Chanock R. M. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol. 1986 Mar;57(3):721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richt J. A., Stitz L., Wekerle H., Rott R. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology in the brain. J Exp Med. 1989 Sep 1;170(3):1045–1050. doi: 10.1084/jem.170.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J. H., O'Garra A., Shrader B., van Kimmenade A., Bond M. W., Mosmann T. R., Coffman R. L. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol. 1988 Sep 1;141(5):1576–1581. [PubMed] [Google Scholar]
- Scott P., Caspar P., Sher A. Protection against Leishmania major in BALB/c mice by adoptive transfer of a T cell clone recognizing a low molecular weight antigen released by promastigotes. J Immunol. 1990 Feb 1;144(3):1075–1079. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Doyle C., Yang Z., Kappes D., Strominger J. L. Structural features of the cytoplasmic region of CD4 required for internalization. EMBO J. 1990 Feb;9(2):425–434. doi: 10.1002/j.1460-2075.1990.tb08127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Taylor G., Ball L. A., Anderson K., Young K. K., King A. M., Wertz G. W. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987 Dec;61(12):3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Bew M., Fernie B. F., Cote P. J., Collins A. P., Hughes M., Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984 May;52(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Taylor P. M., Esquivel F., Askonas B. A. Murine CD4+ T cell clones vary in function in vitro and in influenza infection in vivo. Int Immunol. 1990;2(4):323–328. doi: 10.1093/intimm/2.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldor M. K., Mitchell D., Kipps T. J., Herzenberg L. A., Steinman L. Importance of immunoglobulin isotype in therapy of experimental autoimmune encephalomyelitis with monoclonal anti-CD4 antibody. J Immunol. 1987 Dec 1;139(11):3660–3664. [PubMed] [Google Scholar]
- Wang J. M., Rambaldi A., Biondi A., Chen Z. G., Sanderson C. J., Mantovani A. Recombinant human interleukin 5 is a selective eosinophil chemoattractant. Eur J Immunol. 1989 Apr;19(4):701–705. doi: 10.1002/eji.1830190420. [DOI] [PubMed] [Google Scholar]
- Webb M. S., Henry R. L., Milner A. D., Stokes G. M., Swarbrick A. S. Continuing respiratory problems three and a half years after acute viral bronchiolitis. Arch Dis Child. 1985 Nov;60(11):1064–1067. doi: 10.1136/adc.60.11.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welliver R. C., Kaul A., Ogra P. L. Cell-mediated immune response to respiratory syncytial virus infection: relationship to the development of reactive airway disease. J Pediatr. 1979 Mar;94(3):370–375. doi: 10.1016/s0022-3476(79)80573-9. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Belshe R. B., Kim H. W., Van Voris L. P., Chanock R. M. Administration of a highly attenuated, live respiratory syncytial virus vaccine to adults and children. Infect Immun. 1982 Jul;37(1):397–400. doi: 10.1128/iai.37.1.397-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



