Abstract
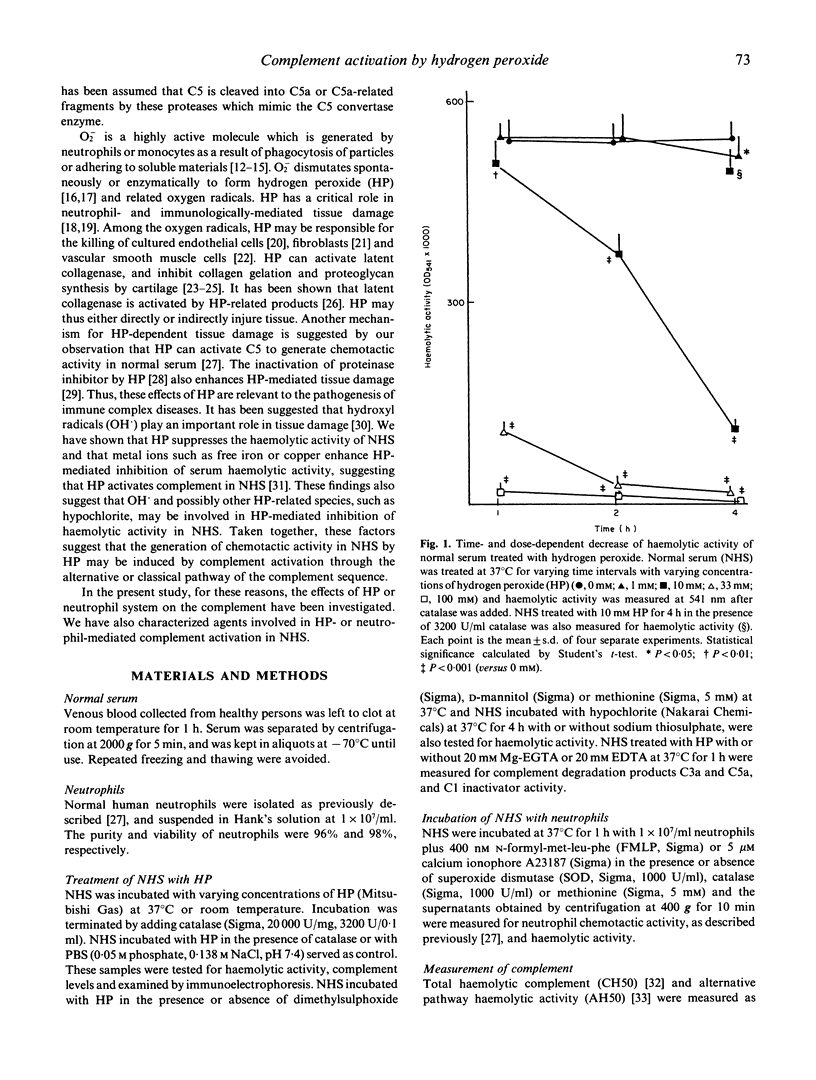
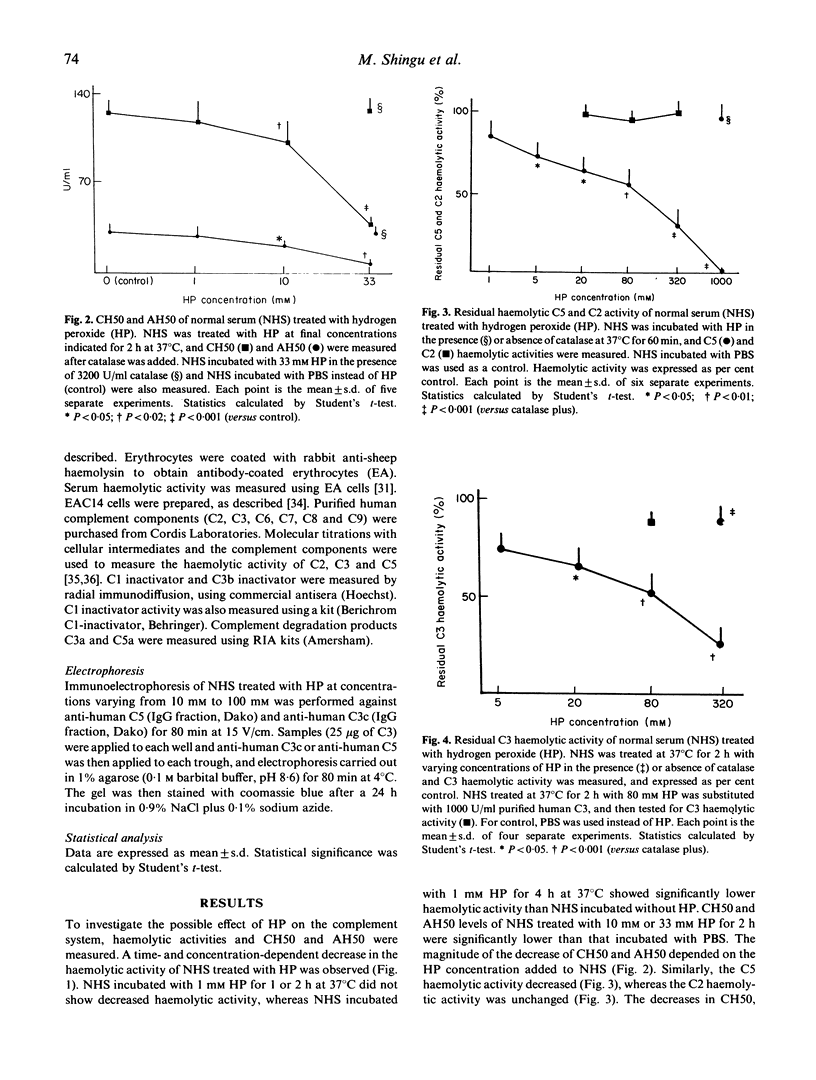
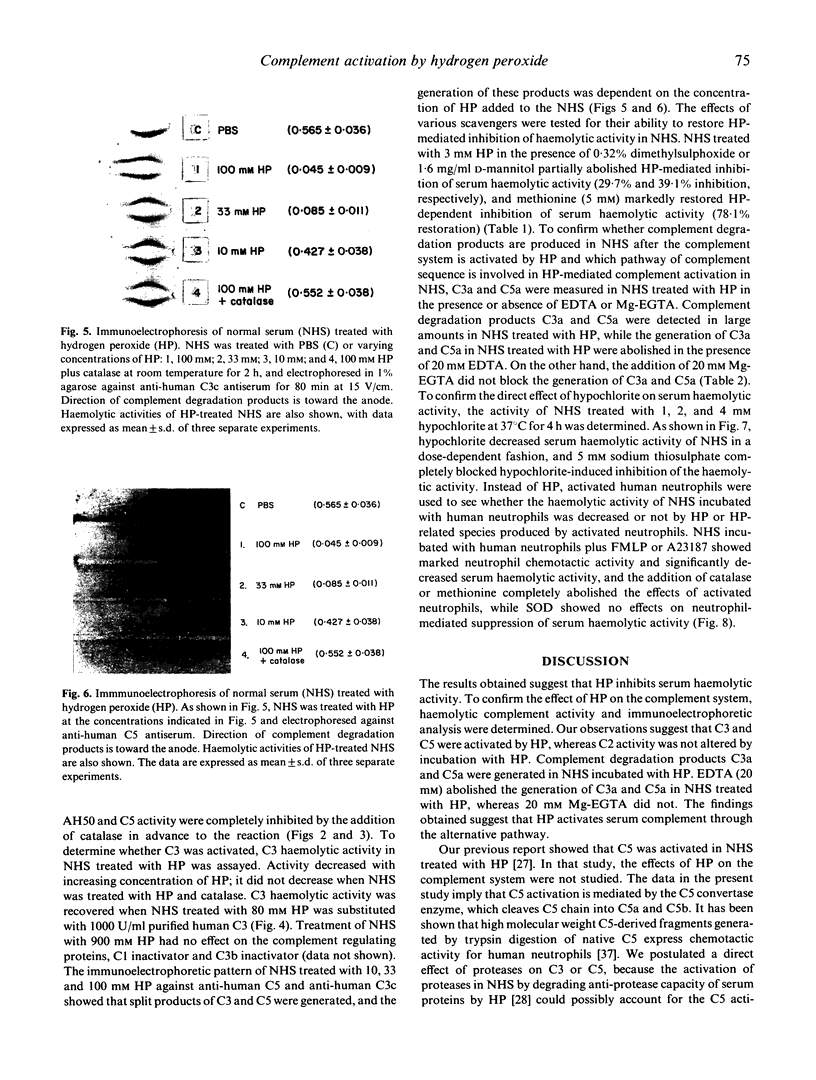
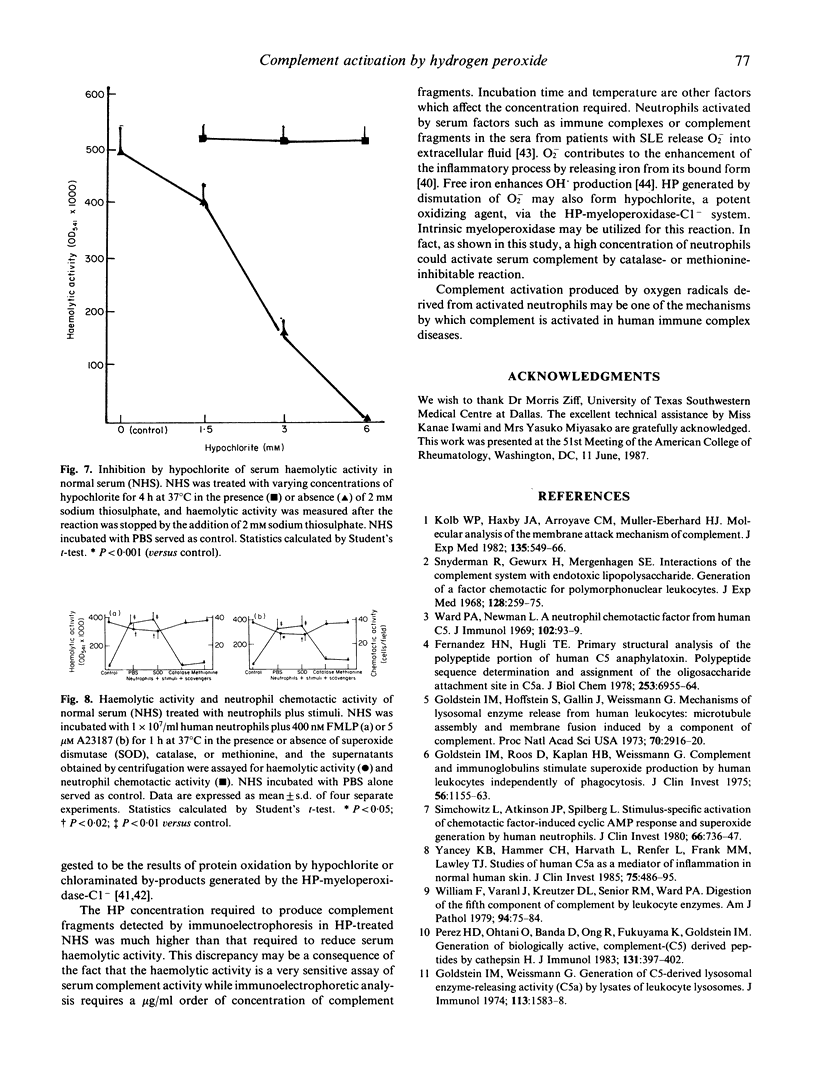
Neutrophils activated by soluble particulate stimuli generate superoxide anion and subsequently form hydrogen peroxide and other oxygen radicals. The effect of hydrogen peroxide on the complement system in normal serum was investigated. Treatment of normal serum with hydrogen peroxide resulted in a diminution of the haemolytic activity of the total and alternative complement pathways and the haemolytic titres of C3 and C5 but not of C2, in normal serum. These decreases in complement activity depended on the concentration of hydrogen peroxide added to the serum. Immunoelectrophoretic analysis of hydrogen peroxide-treated serum showed that C3 and C5 proteins were activated. Complement degradation products C3a and C5a were produced in normal serum treated with hydrogen peroxide, and 20 mM EDTA abolished C3a and C5a production in hydrogen peroxide-treated serum but 20 mM Mg-EGTA did not. Catalase completely abolished and dimethylsulphoxide and D-mannitol, hydroxyl radical scavengers, partially inhibited the hydrogen peroxide-mediated complement activation. Hypochlorite, incubated with normal serum, significantly inhibited serum haemolytic activity, and sodium thiosulphate, a reducing agent, abolished the effect of hypochlorite. Normal serum incubated with activated neutrophils showed neutrophil chemotactic activity and decreased serum haemolytic activity, and the addition of catalase or methionine (5 mM) completely abolished the effects of activated neutrophils. These results suggest that hydrogen peroxide activates complement via an alternative pathway of complement activation and that hydroxyl radicals and other hydrogen peroxide-related species such as hypochlorite are most likely involved in hydrogen peroxide-mediated complement activation. Complement activation by oxygen radicals produced by activated neutrophils may be one of the mechanisms by which complement is activated in human immune complex diseases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmadzadeh N., Shingu M., Nobunaga M. Iron-binding proteins and free iron in synovial fluids of rheumatoid arthritis patients. Clin Rheumatol. 1989 Sep;8(3):345–351. doi: 10.1007/BF02030347. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E. J., Lowther D. A., Handley C. J. Oxygen free-radicals mediate an inhibition of proteoglycan synthesis in cultured articular cartilage. Ann Rheum Dis. 1984 Jun;43(3):462–469. doi: 10.1136/ard.43.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemond P., van Eijk H. G., Swaak A. J., Koster J. F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984 Jun;73(6):1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Invest. 1980 Nov;66(5):987–995. doi: 10.1172/JCI109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Müller-Eberhard H. J. The reaction mechanism of human C5 in immune hemolysis. J Exp Med. 1970 Oct 1;132(4):775–793. doi: 10.1084/jem.132.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Weissmann G. Generation of C5-derived lysosomal enzyme-releasing activity (C5a) by lysates of leukocyte lysosomes. J Immunol. 1974 Nov;113(5):1583–1588. [PubMed] [Google Scholar]
- Goldstein I., Hoffstein S., Gallin J., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes: microtubule assembly and membrane fusion induced by a component of complement. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2916–2920. doi: 10.1073/pnas.70.10.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheum. 1979 Mar;22(3):251–259. doi: 10.1002/art.1780220307. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan-Müller J. W., Weening R. S., Roos D. Production of hydrogen peroxide by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):198–207. [PubMed] [Google Scholar]
- Iida K., Fujita T., Inai S., Sasaki M., Kato T. Complement fixing abilities of IgA myeloma proteins and their fragments: the activation of complement through the classical pathway. Immunochemistry. 1976 Sep;13(9):747–752. doi: 10.1016/0019-2791(76)90195-6. [DOI] [PubMed] [Google Scholar]
- Jasin H. E. Generation of IgG aggregates by the myeloperoxidase-hydrogen peroxide system. J Immunol. 1983 Apr;130(4):1918–1923. [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Johnson R. J., Couser W. G., Chi E. Y., Adler S., Klebanoff S. J. New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1987 May;79(5):1379–1387. doi: 10.1172/JCI112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENT J. F., FIFE E. H., Jr Precise standardization of reagents for complement fixation. Am J Trop Med Hyg. 1963 Jan;12:103–116. doi: 10.4269/ajtmh.1963.12.103. [DOI] [PubMed] [Google Scholar]
- Kolb W. P., Haxby J. A., Arroyave C. M., Müller-Eberhard H. J. Molecular analysis of the membrane attack mechanism of complement. J Exp Med. 1972 Mar 1;135(3):549–566. doi: 10.1084/jem.135.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaki K., Iida K., Inai S. A new method for the preparation of EAC14 cell with human or guinea-pig serum. J Immunol Methods. 1974 Aug;5(3):307–317. doi: 10.1016/0022-1759(74)90117-3. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingu M., Nobunaga M. Chemotactic activity generated in human serum from the fifth component of complement by hydrogen peroxide. Am J Pathol. 1984 Nov;117(2):201–206. [PMC free article] [PubMed] [Google Scholar]
- Shingu M., Nonaka S., Nobunaga M., Ahamadzadeh N. Possible role of H2O2-mediated complement activation and cytokines-mediated fibroblasts superoxide generation on skin inflammation. Dermatologica. 1989;179 (Suppl 1):107–112. doi: 10.1159/000248459. [DOI] [PubMed] [Google Scholar]
- Shingu M., Oribe M., Todoroki T., Tatsukawa K., Tomo-oka K., Yasuda M., Nobunaga M. Serum factors from patients with systemic lupus erythematosus enhancing superoxide generation by normal neutrophils. J Invest Dermatol. 1983 Sep;81(3):212–215. doi: 10.1111/1523-1747.ep12517989. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Atkinson J. P., Spilberg I. Stimulus-specific deactivation of chemotactic factor-induced cyclic AMP response and superoxide generation by human neutrophils. J Clin Invest. 1980 Oct;66(4):736–747. doi: 10.1172/JCI109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Snyderman R., Gewurz H., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide. Generation of a factor chemotactic for polymorphonuclear leukocytes. J Exp Med. 1968 Aug 1;128(2):259–275. doi: 10.1084/jem.128.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Stimulation of a human polymorphonuclear leukocyte oxidative response by the C1q subunit of the first complement component. J Immunol. 1982 Jun;128(6):2547–2552. [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Peppin G., Ortiz X., Ragsdale C., Test S. T. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985 Feb 15;227(4688):747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Regiani S. Neutrophils degrade subendothelial matrices in the presence of alpha-1-proteinase inhibitor. Cooperative use of lysosomal proteinases and oxygen metabolites. J Clin Invest. 1984 May;73(5):1297–1303. doi: 10.1172/JCI111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Ward P. A. Immune complex induced generation of oxygen metabolites by human neutrophils. J Immunol. 1982 Jul;129(1):309–313. [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetsel R. A., Kolb W. P. Complement-independent activation of the fifth component (C5) of human complement: limited trypsin digestion resulting in the expression of biological activity. J Immunol. 1982 May;128(5):2209–2216. [PubMed] [Google Scholar]
- Yancey K. B., Hammer C. H., Harvath L., Renfer L., Frank M. M., Lawley T. J. Studies of human C5a as a mediator of inflammation in normal human skin. J Clin Invest. 1985 Feb;75(2):486–495. doi: 10.1172/JCI111724. [DOI] [PMC free article] [PubMed] [Google Scholar]





