Abstract
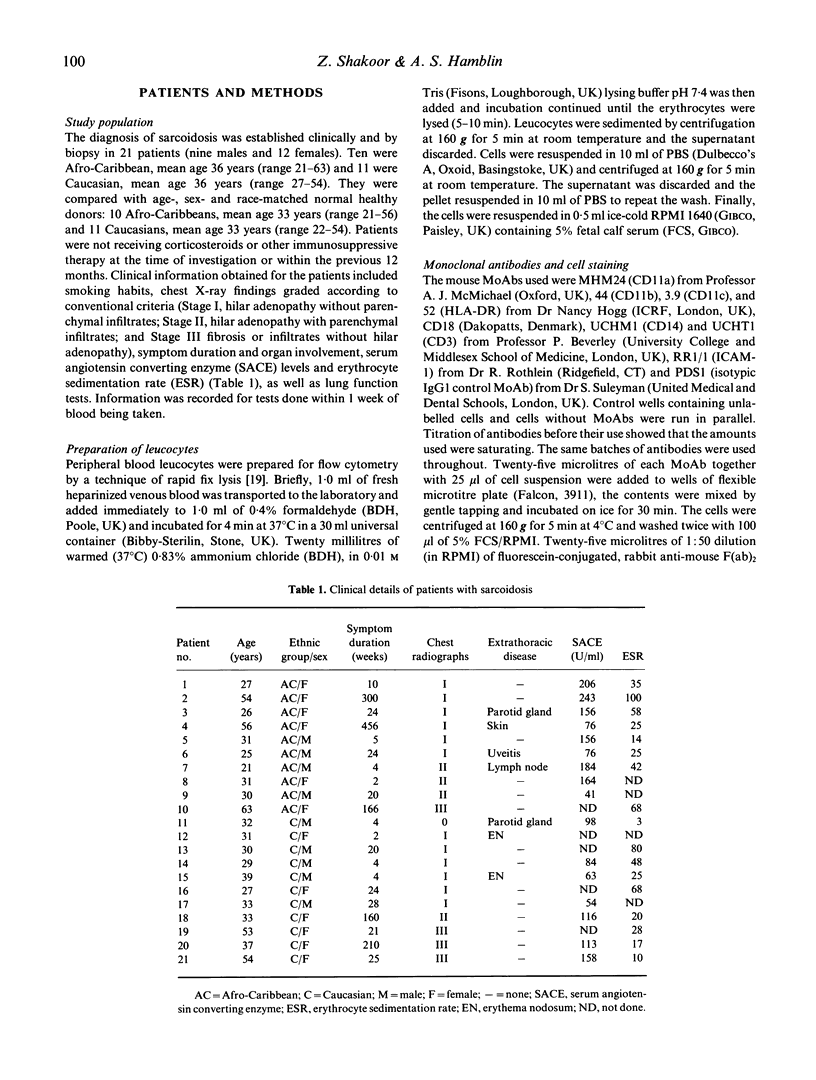
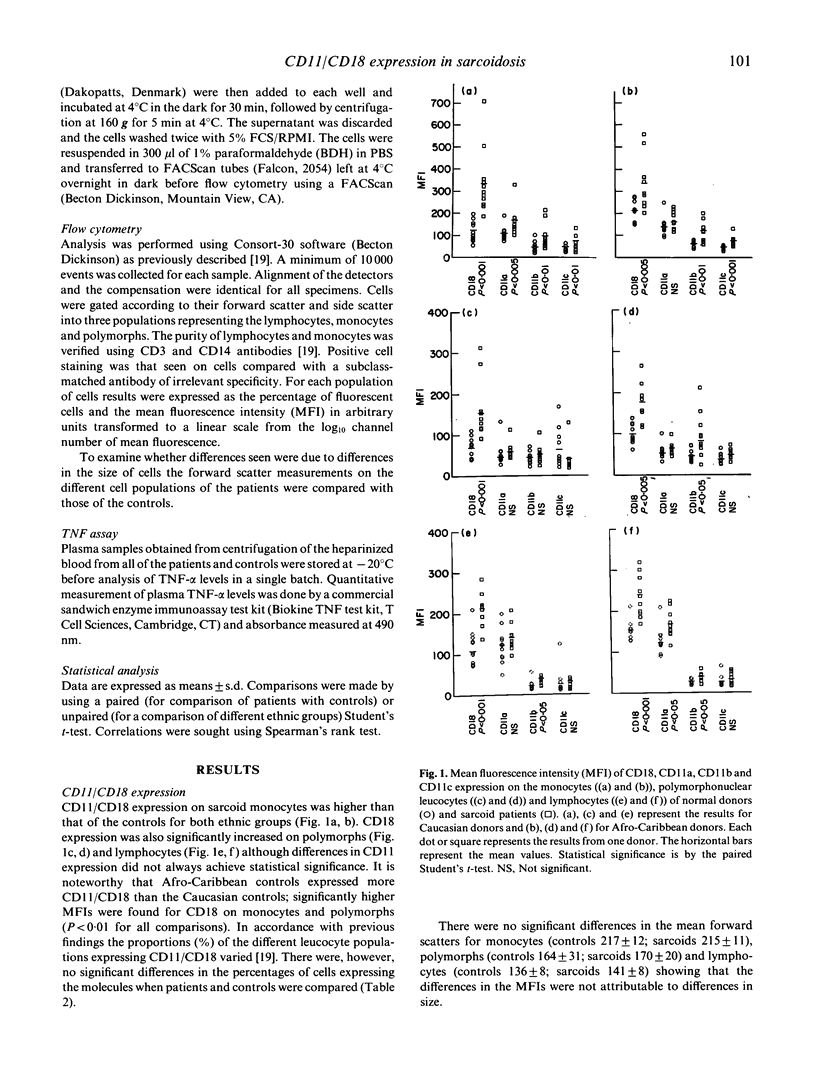
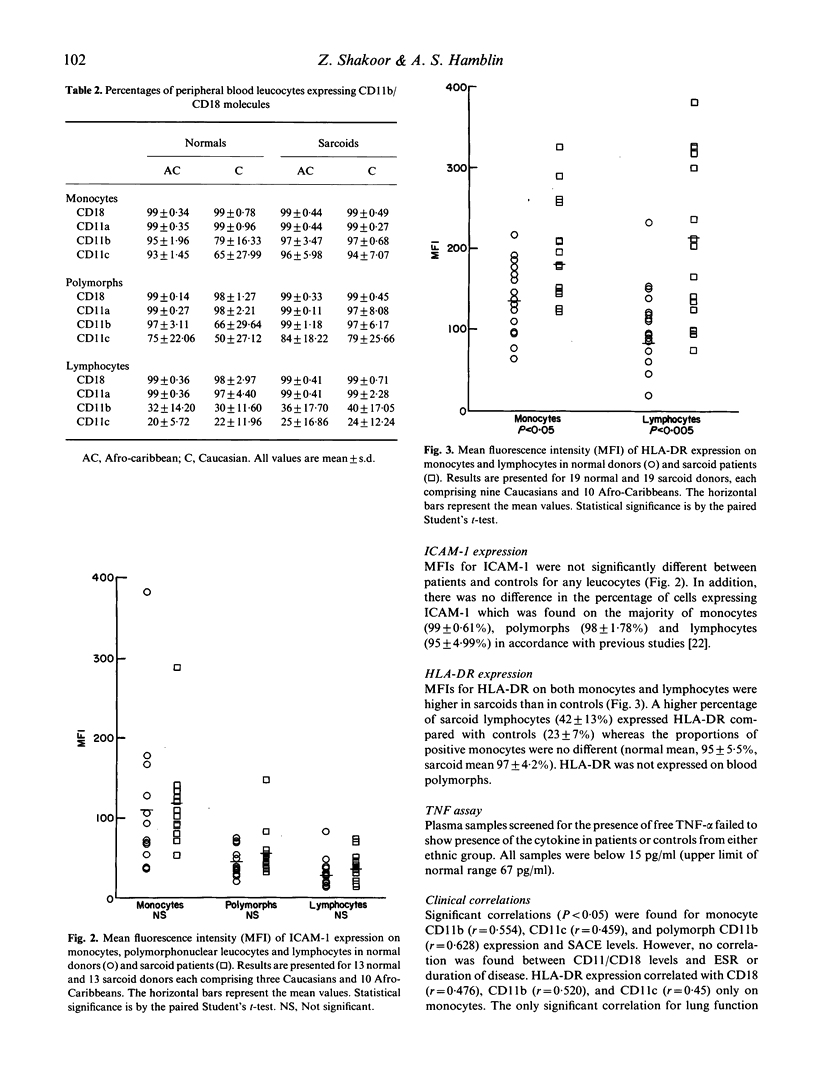
Sarcoidosis is a multisystem disease of unknown etiology characterized by non-caseating granulomata, formed mainly from macrophages surrounded by lymphocytes and plasma cells. Using a novel method for the preparation of blood leucocytes for flow cytometry, we report increased expression of LeuCAMs (CD11/CD18) on peripheral blood leucocytes of 11 Caucasian and 10 Afro-Caribbean patients with sarcoidosis compared with age-, sex- and race-matched controls. Whilst the percentages of the cells expressing CD11/CD18 were no different, the density, expressed as mean fluorescence intensity (MFI), was greater for all leucocytes in sarcoids than in normal individuals. The expression of intercellular adhesion molecule-1 (ICAM-1), a ligand for LFA-1 which is expressed on all leucocytes, was not significantly different from normal, whereas HLA-DR was expressed more intensely on sarcoid monocytes (P less than 0.01) and blood lymphocytes (P less than 0.005) than control cells. Our findings are consistent with leucocyte activation although we were unable to confirm reports of elevated tumour necrosis factor-alpha (TNF-alpha) in the patients' plasma using an ELISA. Increased expression of adhesion molecules on peripheral blood leucocytes may play a role in the cellular extravasation, aggregation, and granuloma formation seen in sarcoidosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Hakim R. M., Todd R. F., 3rd, Dana N., Colten H. R. Increased expression of an adhesion-promoting surface glycoprotein in the granulocytopenia of hemodialysis. N Engl J Med. 1985 Feb 21;312(8):457–462. doi: 10.1056/NEJM198502213120801. [DOI] [PubMed] [Google Scholar]
- Asano M., Minagawa T., Ohmichi M., Hiraga Y. Detection of endogenous cytokines in sera or in lymph nodes obtained from patients with sarcoidosis. Clin Exp Immunol. 1991 Apr;84(1):92–96. doi: 10.1111/j.1365-2249.1991.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa I. L., Gant V. A., Hamblin A. S. Alveolar macrophages from patients with bronchogenic carcinoma and sarcoidosis similarly express monocyte antigens. Clin Exp Immunol. 1991 Oct;86(1):173–178. doi: 10.1111/j.1365-2249.1991.tb05791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrena M. J., Echaniz P., Garcia-Serrano C., Zubillaga P., Cuadrado E. Differential expression of lymphocyte function-associated antigen (LFA-1) on peripheral blood leucocytes from individuals with Down's syndrome. Clin Exp Immunol. 1992 Apr;88(1):41–44. doi: 10.1111/j.1365-2249.1992.tb03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Buyon J. P., Shadick N., Berkman R., Hopkins P., Dalton J., Weissmann G., Winchester R., Abramson S. B. Surface expression of Gp 165/95, the complement receptor CR3, as a marker of disease activity in systemic Lupus erythematosus. Clin Immunol Immunopathol. 1988 Jan;46(1):141–149. doi: 10.1016/0090-1229(88)90014-1. [DOI] [PubMed] [Google Scholar]
- Costabel U., Bross K. J., Rühle K. H., Löhr G. W., Matthys H. Ia-like antigens on T-cells and their subpopulations in pulmonary sarcoidosis and in hypersensitivity pneumonitis. Analysis of bronchoalveolar and blood lymphocytes. Am Rev Respir Dis. 1985 Mar;131(3):337–342. doi: 10.1164/arrd.1985.131.3.337. [DOI] [PubMed] [Google Scholar]
- Danel C., Dewar A., Corrin B., Turner-Warwick M., Chretien J. Ultrastructural changes in bronchoalveolar lavage cells in sarcoidosis and comparison with the tissue granuloma. Am J Pathol. 1983 Jul;112(1):7–17. [PMC free article] [PubMed] [Google Scholar]
- Dougherty G. J., Hogg N. The role of monocyte lymphocyte function-associated antigen 1 (LFA-1) in accessory cell function. Eur J Immunol. 1987 Jul;17(7):943–947. doi: 10.1002/eji.1830170708. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Gant V. A., Shakoor Z., Barbosa I. L., Hamblin A. S. Normal and sarcoid alveolar macrophages differ in their ability to present antigen and to cluster with autologous lymphocytes. Clin Exp Immunol. 1991 Dec;86(3):494–499. doi: 10.1111/j.1365-2249.1991.tb02959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin A., Taylor M., Bernhagen J., Shakoor Z., Mayall S., Noble G., McCarthy D. A method of preparing blood leucocytes for flow cytometry which prevents upregulation of leucocyte integrins. J Immunol Methods. 1992 Feb 5;146(2):219–228. doi: 10.1016/0022-1759(92)90231-h. [DOI] [PubMed] [Google Scholar]
- Hance A. J., Douches S., Winchester R. J., Ferrans V. J., Crystal R. G. Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol. 1985 Jan;134(1):284–292. [PubMed] [Google Scholar]
- Hedfors E., Holm G., Pettersson D. Lymphocyte subpopulations in sarcoidosis. Clin Exp Immunol. 1974 Jun;17(2):219–226. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Kansas G. S., Muirhead M. J., Dailey M. O. Expression of the CD11/CD18, leukocyte adhesion molecule 1, and CD44 adhesion molecules during normal myeloid and erythroid differentiation in humans. Blood. 1990 Dec 15;76(12):2483–2492. [PubMed] [Google Scholar]
- Kürzinger K., Reynolds T., Germain R. N., Davignon D., Martz E., Springer T. A. A novel lymphocyte function-associated antigen (LFA-1): cellular distribution, quantitative expression, and structure. J Immunol. 1981 Aug;127(2):596–602. [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Limb G. A., Hamblin A. S., Wolstencroft R. A., Dumonde D. C. Selective up-regulation of human granulocyte integrins and complement receptor 1 by cytokines. Immunology. 1991 Dec;74(4):696–702. [PMC free article] [PubMed] [Google Scholar]
- McCarthy D., Taylor M. J., Bernhagen J., Perry J. D., Hamblin A. S. Leucocyte integrin and CR1 expression on peripheral blood leucocytes of patients with rheumatoid arthritis. Ann Rheum Dis. 1992 Mar;51(3):307–312. doi: 10.1136/ard.51.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. N., Scadding J. G. Sarcoidosis. Am Rev Respir Dis. 1974 Dec;110(6):774–802. doi: 10.1164/arrd.1974.110.6P1.774. [DOI] [PubMed] [Google Scholar]
- Philip R., Berger A. C., McManus N. H., Warner N. H., Peacock M. A., Epstein L. B. Abnormalities of the in vitro cellular and humoral responses to tetanus and influenza antigens with concomitant numerical alterations in lymphocyte subsets in Down syndrome (trisomy 21). J Immunol. 1986 Mar 1;136(5):1661–1667. [PubMed] [Google Scholar]
- Prieto J., Beatty P. G., Clark E. A., Patarroyo M. Molecules mediating adhesion of T and B cells, monocytes and granulocytes to vascular endothelial cells. Immunology. 1988 Apr;63(4):631–637. [PMC free article] [PubMed] [Google Scholar]
- Salyer J. L., Bohnsack J. F., Knape W. A., Shigeoka A. O., Ashwood E. R., Hill H. R. Mechanisms of tumor necrosis factor-alpha alteration of PMN adhesion and migration. Am J Pathol. 1990 Apr;136(4):831–841. [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Semenzato G., Pezzutto A., Pizzolo G., Chilosi M., Ossi E., Angi M. R., Cipriani A. Immunohistological study in sarcoidosis: evaluation at different sites of disease activity. Clin Immunol Immunopathol. 1984 Jan;30(1):29–40. doi: 10.1016/0090-1229(84)90004-7. [DOI] [PubMed] [Google Scholar]
- Sharma O. P., James D. G., Fox R. A. A correlation of in vivo delayed-type hypersensitivity with in vitro lymphocyte transformation in sarccidosis. Chest. 1971 Jul;60(1):35–37. doi: 10.1378/chest.60.1.35. [DOI] [PubMed] [Google Scholar]
- Siltzbach L. E., James D. G., Neville E., Turiaf J., Battesti J. P., Sharma O. P., Hosoda Y., Mikami R., Odaka M. Course and prognosis of sarcoidosis around the world. Am J Med. 1974 Dec;57(6):847–852. doi: 10.1016/0002-9343(74)90160-0. [DOI] [PubMed] [Google Scholar]
- Taylor G. M., Haigh H., Williams A., D'Souza S. W., Harris R. Down's syndrome lymphoid cell lines exhibit increased adhesion due to the over-expression of lymphocyte function-associated antigen (LFA-1). Immunology. 1988 Jul;64(3):451–456. [PMC free article] [PubMed] [Google Scholar]
- Theilmann L., Meyer U., Kommerell B., Dierkesmann R., Möller A. Tumor-Nekrose-Faktor-Alpha im Serum von Patienten mit Sarkoidose, Tuberkulose oder Bronchialkarzinom. Pneumologie. 1990 Apr;44(4):735–738. [PubMed] [Google Scholar]
- Vedder N. B., Harlan J. M. Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest. 1988 Mar;81(3):676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venet A., Hance A. J., Saltini C., Robinson B. W., Crystal R. G. Enhanced alveolar macrophage-mediated antigen-induced T-lymphocyte proliferation in sarcoidosis. J Clin Invest. 1985 Jan;75(1):293–301. doi: 10.1172/JCI111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw A. Leucocyte adhesion to endothelium. Clin Exp Allergy. 1990 Nov;20(6):619–626. doi: 10.1111/j.1365-2222.1990.tb02700.x. [DOI] [PubMed] [Google Scholar]



