Abstract
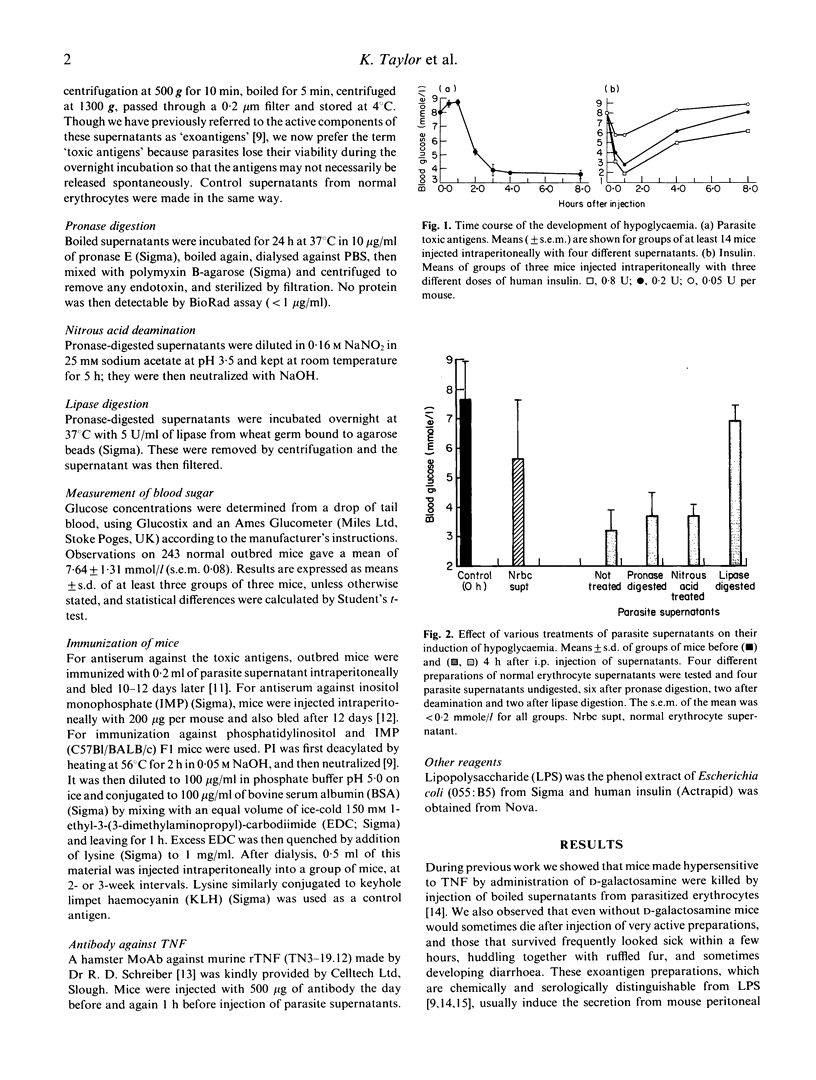
Hypoglycaemia is associated with severe malaria and is an important prognostic indicator. Molecules liberated during overnight incubation of erythrocytes infected with Plasmodium yoelii induce marked hypoglycaemia in normal mice, with a delayed time course compared with insulin; some, though weaker, activity could also be obtained by overnight incubation of uninfected erythrocytes. The active component shares many properties with the phospholipid-containing molecules which we have previously shown to be toxic and to induce the release of tumour necrosis factor (TNF) from macrophages. However a MoAb which neutralizes the cytotoxicity of tumour necrosis factor in vitro did not prevent this induction of hypoglycaemia, whereas antiserum against the toxic antigens did, as did immunization of normal (but not the immunoglobulin-deficient SCID) mice with the same material. Furthermore, normal mice injected with the antigens after immunization with phosphatidyl inositol or inositol monophosphate did not develop hypoglycaemia; the latter compound was also inhibitory when mixed with the antigens before injection. These compounds were previously shown to block the induction of TNF by the antigens and to induce the production of inhibitory antibodies. The role of these molecules in the etiology of the hypoglycaemia of malaria is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bate C. A., Taverne J., Bootsma H. J., Mason R. C., Skalko N., Gregoriadis G., Playfair J. H. Antibodies against phosphatidylinositol and inositol monophosphate specifically inhibit tumour necrosis factor induction by malaria exoantigens. Immunology. 1992 May;76(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Davé A., Playfair J. H. Malaria exoantigens induce T-independent antibody that blocks their ability to induce TNF. Immunology. 1990 Jul;70(3):315–320. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Detoxified exoantigens and phosphatidylinositol derivatives inhibit tumor necrosis factor induction by malarial exoantigens. Infect Immun. 1992 May;60(5):1894–1901. doi: 10.1128/iai.60.5.1894-1901.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
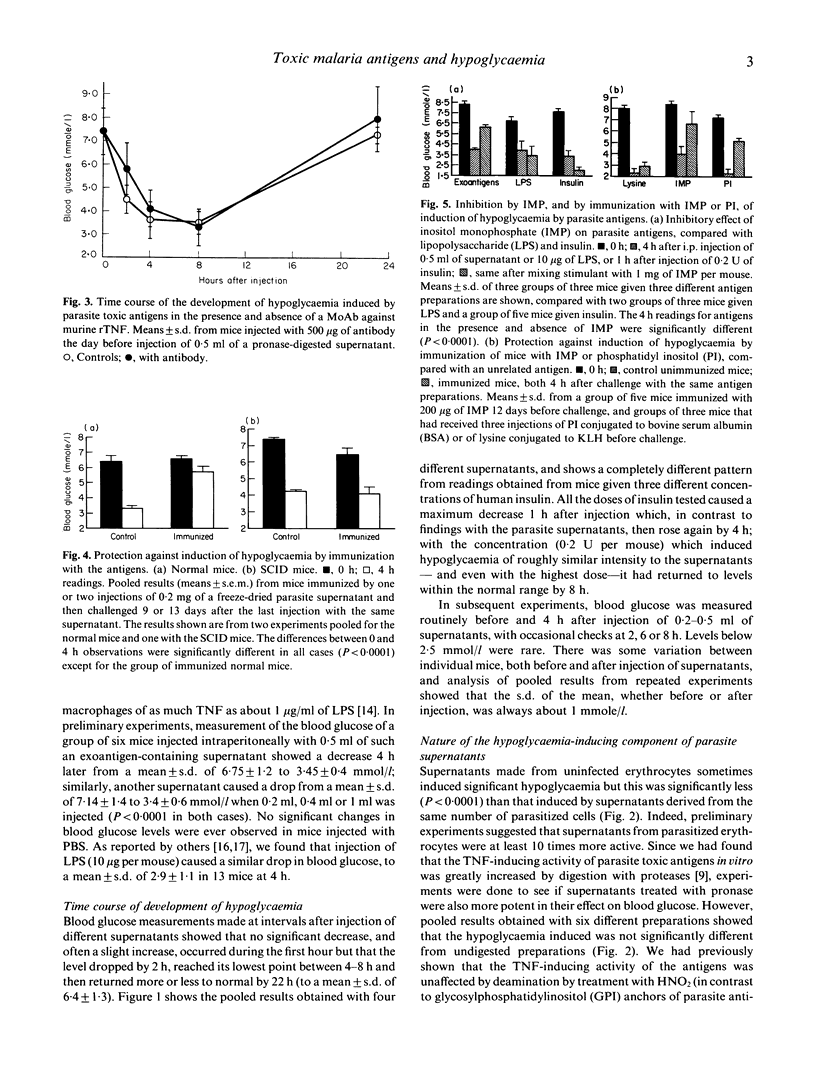
- Bate C. A., Taverne J., Playfair J. H. Soluble malarial antigens are toxic and induce the production of tumour necrosis factor in vivo. Immunology. 1989 Apr;66(4):600–605. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Román E., Moreno C., Playfair J. H. Tumour necrosis factor induction by malaria exoantigens depends upon phospholipid. Immunology. 1992 Jan;75(1):129–135. [PMC free article] [PubMed] [Google Scholar]
- Bauss F., Dröge W., Männel D. N. Tumor necrosis factor mediates endotoxic effects in mice. Infect Immun. 1987 Jul;55(7):1622–1625. doi: 10.1128/iai.55.7.1622-1625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Brewster D. R., Kwiatkowski D., White N. J. Neurological sequelae of cerebral malaria in children. Lancet. 1990 Oct 27;336(8722):1039–1043. doi: 10.1016/0140-6736(90)92498-7. [DOI] [PubMed] [Google Scholar]
- Clark I. A. Cell-mediated immunity in protection and pathology of malaria. Parasitol Today. 1987 Oct;3(10):300–305. doi: 10.1016/0169-4758(87)90187-6. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Taylor T. E., Molyneux M. E., Wirima J. J., Vassalli P., Hommel M., Lambert P. H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989 Jun 15;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Greenwood B., Marsh K., Snow R. Why do some African children develop severe malaria? Parasitol Today. 1991 Oct;7(10):277–281. doi: 10.1016/0169-4758(91)90096-7. [DOI] [PubMed] [Google Scholar]
- Holloway P. A., Krishna S., White N. J. Plasmodium berghei: lactic acidosis and hypoglycaemia in a rodent model of severe malaria; effects of glucose, quinine, and dichloroacetate. Exp Parasitol. 1991 Feb;72(2):123–133. doi: 10.1016/0014-4894(91)90130-o. [DOI] [PubMed] [Google Scholar]
- Kern P., Hemmer C. J., Van Damme J., Gruss H. J., Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989 Aug;87(2):139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Hill A. V., Sambou I., Twumasi P., Castracane J., Manogue K. R., Cerami A., Brewster D. R., Greenwood B. M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990 Nov 17;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- Machicao F., Mushack J., Seffer E., Ermel B., Häring H. U. Mannose, glucosamine and inositol monophosphate inhibit the effects of insulin on lipogenesis. Further evidence for a role for inositol phosphate-oligosaccharides in insulin action. Biochem J. 1990 Mar 15;266(3):909–916. [PMC free article] [PubMed] [Google Scholar]
- Maguire P. A., Prudhomme J., Sherman I. W. Alterations in erythrocyte membrane phospholipid organization due to the intracellular growth of the human malaria parasite, Plasmodium falciparum. Parasitology. 1991 Apr;102(Pt 2):179–186. doi: 10.1017/s0031182000062466. [DOI] [PubMed] [Google Scholar]
- McNeil H. P., Simpson R. J., Chesterman C. N., Krilis S. A. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H). Proc Natl Acad Sci U S A. 1990 Jun;87(11):4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux M. E., Taylor T. E., Wirima J. J., Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989 May;71(265):441–459. [PubMed] [Google Scholar]
- Nimpf J., Bevers E. M., Bomans P. H., Till U., Wurm H., Kostner G. M., Zwaal R. F. Prothrombinase activity of human platelets is inhibited by beta 2-glycoprotein-I. Biochim Biophys Acta. 1986 Oct 29;884(1):142–149. doi: 10.1016/0304-4165(86)90237-0. [DOI] [PubMed] [Google Scholar]
- Sepehrnia B., Kamboh M. I., Adams-Campbell L. L., Bunker C. H., Nwankwo M., Majumder P. P., Ferrell R. E. Genetic studies of human apolipoproteins. VIII. Role of the apolipoprotein H polymorphism in relation to serum lipoprotein concentrations. Hum Genet. 1989 May;82(2):118–122. doi: 10.1007/BF00284041. [DOI] [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Vogel S. N., Havell E. A. Differential inhibition of lipopolysaccharide-induced phenomena by anti-tumor necrosis factor alpha antibody. Infect Immun. 1990 Jul;58(7):2397–2400. doi: 10.1128/iai.58.7.2397-2400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Henricson B. E., Neta R. Roles of interleukin-1 and tumor necrosis factor in lipopolysaccharide-induced hypoglycemia. Infect Immun. 1991 Jul;59(7):2494–2498. doi: 10.1128/iai.59.7.2494-2498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey A., Besedovsky H. Interleukin 1 affects glucose homeostasis. Am J Physiol. 1987 Nov;253(5 Pt 2):R794–R798. doi: 10.1152/ajpregu.1987.253.5.R794. [DOI] [PubMed] [Google Scholar]


