Abstract
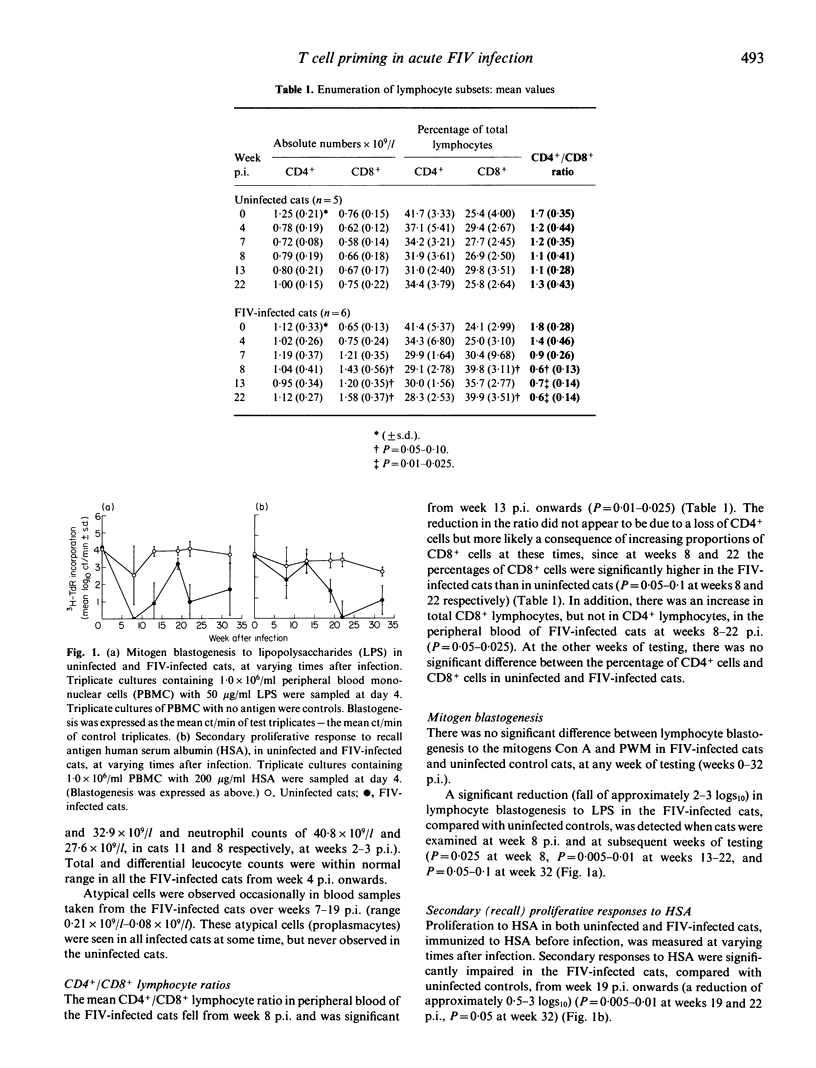
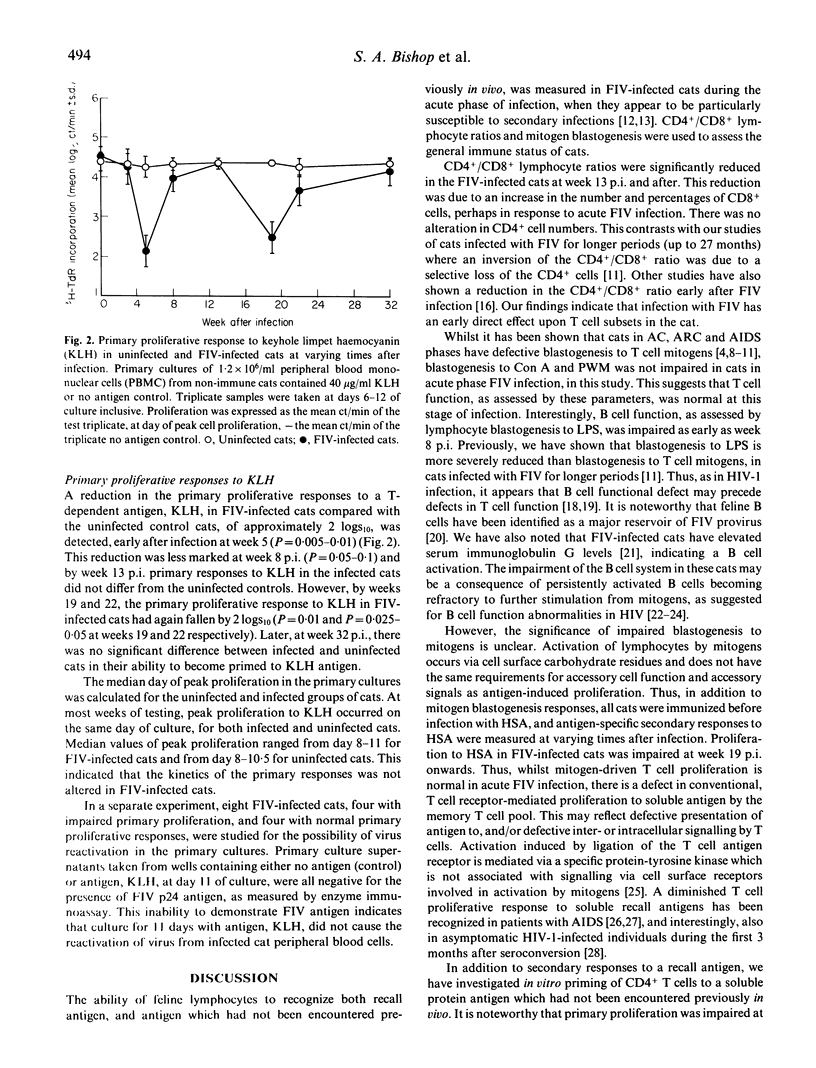
As in HIV infection of humans, cats infected with FIV are particularly susceptible to secondary infection by opportunistic pathogens, suggesting an impaired ability to elicit an effective immune response against foreign antigens. In order to investigate the development of immunity in FIV-infected cats, we have used an autologous culture system to directly measure priming of naive CD4+ T cells to soluble protein antigen, in vitro. Using this assay, we showed previously that cats infected with FIV for several months had significantly reduced primary proliferative responses. We have now examined cats before infection, and at varying times after infection with FIV, to determine how soon after infection this defect in T cell priming was evident, compared with other quantitative and qualitative measurements of lymphocyte function. Our results showed a progressive decline in immune function in asymptomatic cats during the acute stage of infection with FIV. Primary T cell responses were most sensitive and a significant reduction in proliferation of naive T cells to foreign antigen occurred 5 weeks after infection, despite normal blastogenesis to T cell mitogens and normal CD4+/CD8+ ratios at these times. Whilst lymphocyte proliferation to T cell mitogens was unaffected throughout, a significant reduction in proliferation to a B cell mitogen occurred from week 8 onwards. CD4+/CD8+ ratios fell significantly from week 13 onwards, and proliferation of the memory T cell population to a recall antigen was significantly impaired later, from week 19 onwards. The defect in the priming of naive T cells to foreign antigen early after infection may be important in determining susceptibility to secondary infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackley C. D., Hoover E. A., Cooper M. D. Identification of a CD4 homologue in the cat. Tissue Antigens. 1990 Feb;35(2):92–98. doi: 10.1111/j.1399-0039.1990.tb01762.x. [DOI] [PubMed] [Google Scholar]
- Amadori A., Zamarchi R., Ciminale V., Del Mistro A., Siervo S., Alberti A., Colombatti M., Chieco-Bianchi L. HIV-1-specific B cell activation. A major constituent of spontaneous B cell activation during HIV-1 infection. J Immunol. 1989 Oct 1;143(7):2146–2152. [PubMed] [Google Scholar]
- Bishop S. A., Williams N. A., Gruffydd-Jones T. J., Harbour D. A., Stokes C. R. Impaired T-cell priming and proliferation in cats infected with feline immunodeficiency virus. AIDS. 1992 Mar;6(3):287–293. doi: 10.1097/00002030-199203000-00005. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Bissey L., Logan K. S., Pedersen N. C., Elder J. H., Collisson E. W. Feline immunodeficiency virus infects both CD4+ and CD8+ T lymphocytes. J Virol. 1991 Jun;65(6):3359–3364. doi: 10.1128/jvi.65.6.3359-3364.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D., Pedersen N. C. Infection of peritoneal macrophages in vitro and in vivo with feline immunodeficiency virus. J Virol. 1989 Dec;63(12):5483–5488. doi: 10.1128/jvi.63.12.5483-5488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers S., Schick R. O., Jeffers J. Demodicosis in two cats seropositive for feline immunodeficiency virus. J Am Vet Med Assoc. 1989 Jan 15;194(2):256–257. [PubMed] [Google Scholar]
- Dawson S., Smyth N. R., Bennett M., Gaskell R. M., McCracken C. M., Brown A., Gaskell C. J. Effect of primary-stage feline immunodeficiency virus infection on subsequent feline calicivirus vaccination and challenge in cats. AIDS. 1991 Jun;5(6):747–750. doi: 10.1097/00002030-199106000-00016. [DOI] [PubMed] [Google Scholar]
- Ennen J., Seipp I., Norley S. G., Kurth R. Decreased accessory cell function of macrophages after infection with human immunodeficiency virus type 1 in vitro. Eur J Immunol. 1990 Nov;20(11):2451–2456. doi: 10.1002/eji.1830201114. [DOI] [PubMed] [Google Scholar]
- Epstein J. S., Frederick W. R., Rook A. H., Jackson L., Manischewitz J. F., Mayner R. E., Masur H., Enterline J. C., Djeu J. Y., Quinnan G. V., Jr Selective defects in cytomegalovirus- and mitogen-induced lymphocyte proliferation and interferon release in patients with acquired immunodeficiency syndrome. J Infect Dis. 1985 Oct;152(4):727–733. doi: 10.1093/infdis/152.4.727. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Hara Y., Ishida T., Ejima H., Tagawa M., Motoyoshi S., Tomoda I., Shimizu M., Shichinohe K. Decrease in mitogen-induced lymphocyte proliferative responses in cats infected with feline immunodeficiency virus. Nihon Juigaku Zasshi. 1990 Jun;52(3):573–579. doi: 10.1292/jvms1939.52.573. [DOI] [PubMed] [Google Scholar]
- Hopper C. D., Sparkes A. H., Gruffydd-Jones T. J., Crispin S. M., Muir P., Harbour D. A., Stokes C. R. Clinical and laboratory findings in cats infected with feline immunodeficiency virus. Vet Rec. 1989 Sep 23;125(13):341–346. doi: 10.1136/vr.125.13.341. [DOI] [PubMed] [Google Scholar]
- Katz I. R., Krown S. E., Safai B., Oettgen H. F., Hoffmann M. K. Antigen-specific and polyclonal B-cell responses in patients with acquired immunodeficiency disease syndrome. Clin Immunol Immunopathol. 1986 Jun;39(3):359–367. doi: 10.1016/0090-1229(86)90164-9. [DOI] [PubMed] [Google Scholar]
- Klotz F. W., Cooper M. D. A feline thymocyte antigen defined by a monoclonal antibody (FT2) identifies a subpopulation of non-helper cells capable of specific cytotoxicity. J Immunol. 1986 Apr 1;136(7):2510–2514. [PubMed] [Google Scholar]
- Lane H. C., Depper J. M., Greene W. C., Whalen G., Waldmann T. A., Fauci A. S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985 Jul 11;313(2):79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- Lin D. S., Bowman D. D., Jacobson R. H., Barr M. C., Fevereiro M., Williams J. R., Noronha F. M., Scott F. W., Avery R. J. Suppression of lymphocyte blastogenesis to mitogens in cats experimentally infected with feline immunodeficiency virus. Vet Immunol Immunopathol. 1990 Oct;26(2):183–189. doi: 10.1016/0165-2427(90)90066-2. [DOI] [PubMed] [Google Scholar]
- Macatonia S. E., Lau R., Patterson S., Pinching A. J., Knight S. C. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990 Sep;71(1):38–45. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Taylor P. M., Knight S. C., Askonas B. A. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989 Apr 1;169(4):1255–1264. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedema F., Petit A. J., Terpstra F. G., Schattenkerk J. K., de Wolf F., Al B. J., Roos M., Lange J. M., Danner S. A., Goudsmit J. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Invest. 1988 Dec;82(6):1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotney C., English R. V., Housman J., Davidson M. G., Nasisse M. P., Jeng C. R., Davis W. C., Tompkins M. B. Lymphocyte population changes in cats naturally infected with feline immunodeficiency virus. AIDS. 1990 Dec;4(12):1213–1218. doi: 10.1097/00002030-199012000-00005. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Yamamoto J. K., Ishida T., Hansen H. Feline immunodeficiency virus infection. Vet Immunol Immunopathol. 1989 May;21(1):111–129. doi: 10.1016/0165-2427(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Ranki A., Valle S. L., Krohn M., Antonen J., Allain J. P., Leuther M., Franchini G., Krohn K. Long latency precedes overt seroconversion in sexually transmitted human-immunodeficiency-virus infection. Lancet. 1987 Sep 12;2(8559):589–593. doi: 10.1016/s0140-6736(87)92985-0. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Phillips A. F., Luong E. T., Klausner R. D. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4358–4362. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebelink K. H., Chu I. H., Rimmelzwaan G. F., Weijer K., van Herwijnen R., Knell P., Egberink H. F., Bosch M. L., Osterhaus A. D. Feline immunodeficiency virus (FIV) infection in the cat as a model for HIV infection in man: FIV-induced impairment of immune function. AIDS Res Hum Retroviruses. 1990 Dec;6(12):1373–1378. doi: 10.1089/aid.1990.6.1373. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Teeuwsen V. J., Siebelink K. H., de Wolf F., Goudsmit J., UytdeHaag F. G., Osterhaus A. D. Impairment of in vitro immune responses occurs within 3 months after HIV-1 seroconversion. AIDS. 1990 Jan;4(1):77–81. doi: 10.1097/00002030-199001000-00011. [DOI] [PubMed] [Google Scholar]
- Terpstra F. G., Al B. J., Roos M. T., De Wolf F., Goudsmit J., Schellekens P. T., Miedema F. Longitudinal study of leukocyte functions in homosexual men seroconverted for HIV: rapid and persistent loss of B cell function after HIV infection. Eur J Immunol. 1989 Apr;19(4):667–673. doi: 10.1002/eji.1830190415. [DOI] [PubMed] [Google Scholar]
- Witt C. J., Moench T. R., Gittelsohn A. M., Bishop B. D., Childs J. E. Epidemiologic observations on feline immunodeficiency virus and Toxoplasma gondii coinfection in cats in Baltimore, Md. J Am Vet Med Assoc. 1989 Jan 15;194(2):229–233. [PubMed] [Google Scholar]
- Yamamoto J. K., Hansen H., Ho E. W., Morishita T. Y., Okuda T., Sawa T. R., Nakamura R. M., Pedersen N. C. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc. 1989 Jan 15;194(2):213–220. [PubMed] [Google Scholar]
- Yamamoto J. K., Sparger E., Ho E. W., Andersen P. R., O'Connor T. P., Mandell C. P., Lowenstine L., Munn R., Pedersen N. C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988 Aug;49(8):1246–1258. [PubMed] [Google Scholar]



