Abstract
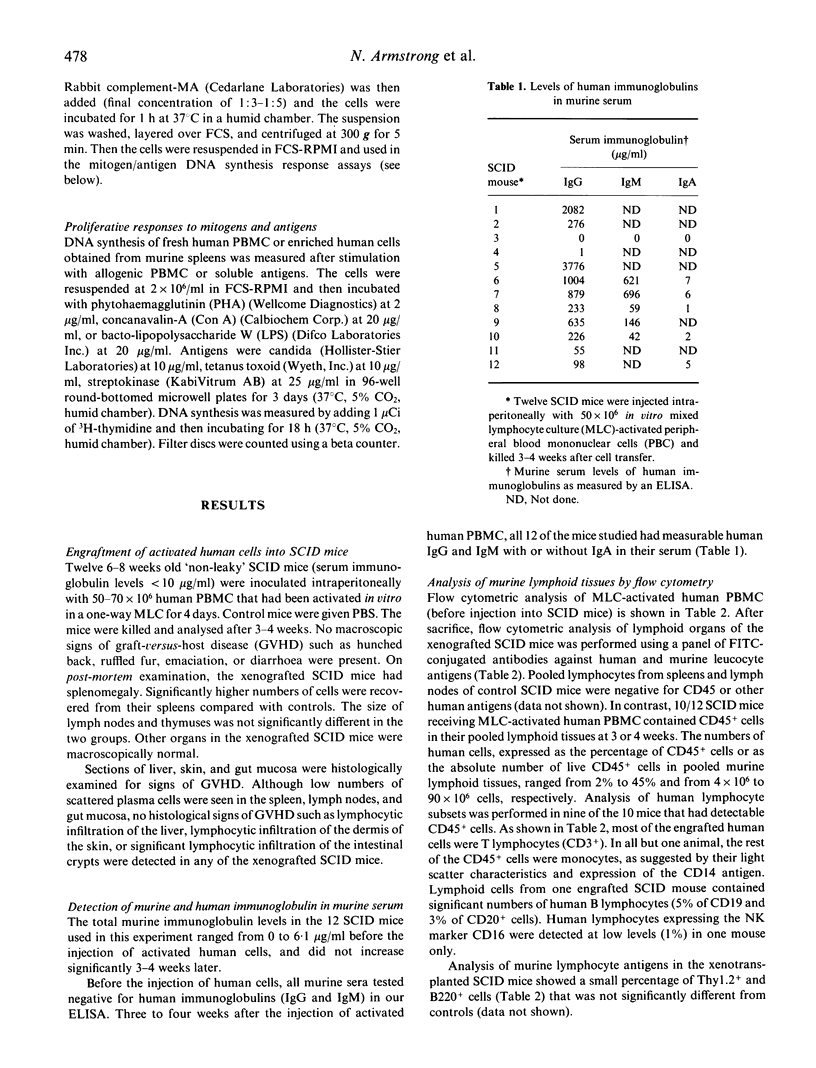
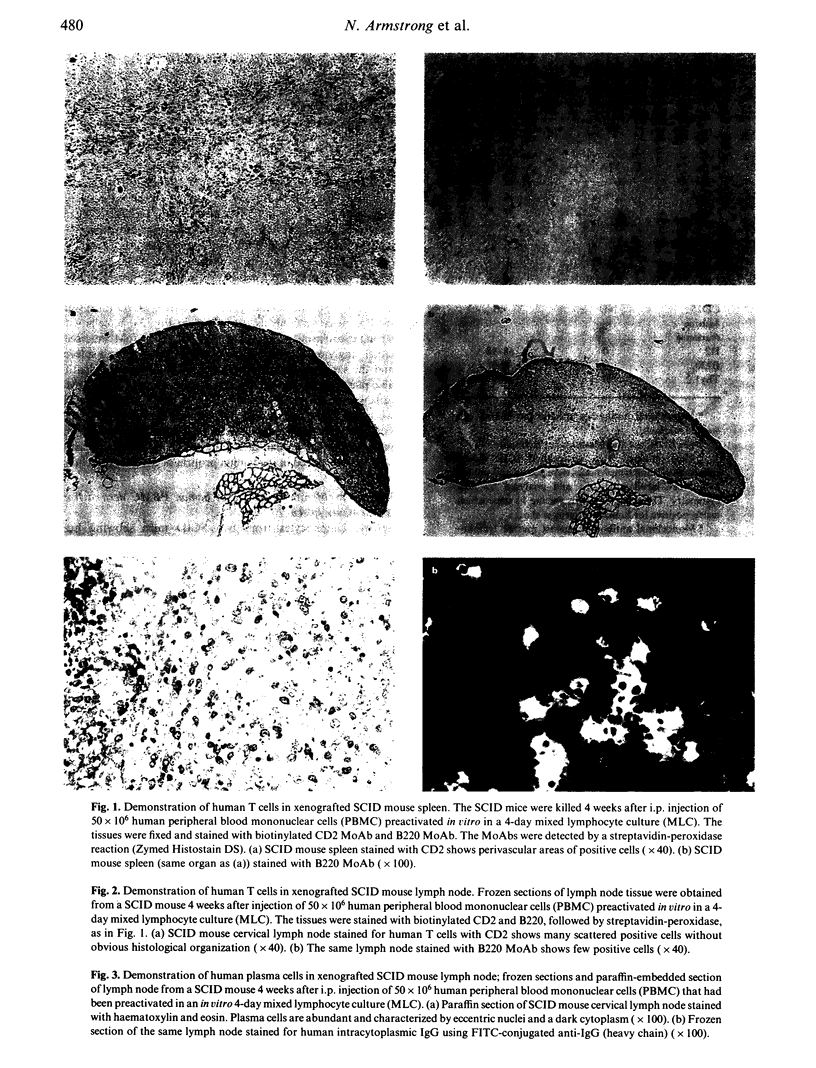
Mice with severe combined immunodeficiency (SCID) accept grafts of human T and B lymphocytes derived from resting peripheral blood mononuclear cells (PBMC). We wished to determine whether activated human T cells engraft and migrate into lymphoid tissues in SCID mice. PBMC (50 x 10(6)) activated in vitro in a 4-day mixed lymphocyte culture (MLC) were injected into the peritoneum of 12 SCID mice. In 11 of 12 animals killed at 3 or 4 weeks after injection, human cells were detected in cells pooled from lymphoid organs by flow cytometry and by immunohistochemical staining of frozen tissue sections. The percentage of CD45+ cells in the 11 mice ranged from 2% to 45% and the absolute numbers of CD45+ cells recovered from lymphoid organs ranged from 4 x 10(6) to 90 x 10(6). Up to 93% of the human cells expressed the CD3 antigen together with either CD4 or CD8. Human T cells were localized in periarteriolar areas in murine spleens, whereas in the lymph nodes and gut mucosa, the T cells did not show the pattern for T-dependent areas found in human lymphoid tissue. Numerous human plasma cells were detected in the spleen and gut mucosal crypts of engrafted SCID mice. Human IgG was detected in the serum of all 11 engrafted SCID mice. The functional activity of human T cells recovered from murine splenic tissue was very low 3-4 weeks after engraftment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry T. S., Jones D. M., Richter C. B., Haynes B. F. Successful engraftment of human postnatal thymus in severe combined immune deficient (SCID) mice: differential engraftment of thymic components with irradiation versus anti-asialo GM-1 immunosuppressive regimens. J Exp Med. 1991 Jan 1;173(1):167–180. doi: 10.1084/jem.173.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Cannon M. J., Pisa P., Fox R. I., Cooper N. R. Epstein-Barr virus induces aggressive lymphoproliferative disorders of human B cell origin in SCID/hu chimeric mice. J Clin Invest. 1990 Apr;85(4):1333–1337. doi: 10.1172/JCI114573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990 Oct 4;347(6292):479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- Helzlsouer K. J., Gordon G. B., Alberg A. J., Bush T. L., Comstock G. W. Relationship of prediagnostic serum levels of dehydroepiandrosterone and dehydroepiandrosterone sulfate to the risk of developing premenopausal breast cancer. Cancer Res. 1992 Jan 1;52(1):1–4. [PubMed] [Google Scholar]
- Kamel-Reid S., Letarte M., Sirard C., Doedens M., Grunberger T., Fulop G., Freedman M. H., Phillips R. A., Dick J. E. A model of human acute lymphoblastic leukemia in immune-deficient SCID mice. Science. 1989 Dec 22;246(4937):1597–1600. doi: 10.1126/science.2595371. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Niguma T., Fechner J. H., Jr, Wolber R., Beeskau M. A., Hullett D. A., Sollinger H. W., Burlingham W. J. Chronic human skin graft rejection in severe combined immunodeficient mice engrafted with human PBL from an HLA-presensitized donor. Transplantation. 1992 Mar;53(3):659–665. doi: 10.1097/00007890-199203000-00032. [DOI] [PubMed] [Google Scholar]
- Krams S. M., Dorshkind K., Gershwin M. E. Generation of biliary lesions after transfer of human lymphocytes into severe combined immunodeficient (SCID) mice. J Exp Med. 1989 Dec 1;170(6):1919–1930. doi: 10.1084/jem.170.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krowka J. F., Sarin S., Namikawa R., McCune J. M., Kaneshima H. Human T cells in the SCID-hu mouse are phenotypically normal and functionally competent. J Immunol. 1991 Jun 1;146(11):3751–3756. [PubMed] [Google Scholar]
- Lubin I., Faktorowich Y., Lapidot T., Gan Y., Eshhar Z., Gazit E., Levite M., Reisner Y. Engraftment and development of human T and B cells in mice after bone marrow transplantation. Science. 1991 Apr 19;252(5004):427–431. doi: 10.1126/science.1826797. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Shih C. C., Rabin L., Kaneshima H. Suppression of HIV infection in AZT-treated SCID-hu mice. Science. 1990 Feb 2;247(4942):564–566. doi: 10.1126/science.2300816. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B., Spector D. H., Spector S. A. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991 Feb 15;251(4995):791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Mosier D. E. Immunodeficient mice xenografted with human lymphoid cells: new models for in vivo studies of human immunobiology and infectious diseases. J Clin Immunol. 1990 Jul;10(4):185–191. doi: 10.1007/BF00918650. [DOI] [PubMed] [Google Scholar]
- Mueller B. M., Romerdahl C. A., Trent J. M., Reisfeld R. A. Suppression of spontaneous melanoma metastasis in scid mice with an antibody to the epidermal growth factor receptor. Cancer Res. 1991 Apr 15;51(8):2193–2198. [PubMed] [Google Scholar]
- Rowe M., Young L. S., Crocker J., Stokes H., Henderson S., Rickinson A. B. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med. 1991 Jan 1;173(1):147–158. doi: 10.1084/jem.173.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Wolf I. G., Negrin R. S., Kiem H. P., Blume K. G., Weissman I. L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991 Jul 1;174(1):139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tary-Lehmann M., Saxon A. Human mature T cells that are anergic in vivo prevail in SCID mice reconstituted with human peripheral blood. J Exp Med. 1992 Feb 1;175(2):503–516. doi: 10.1084/jem.175.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]






