Abstract
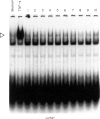
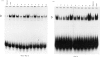
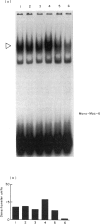
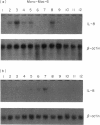
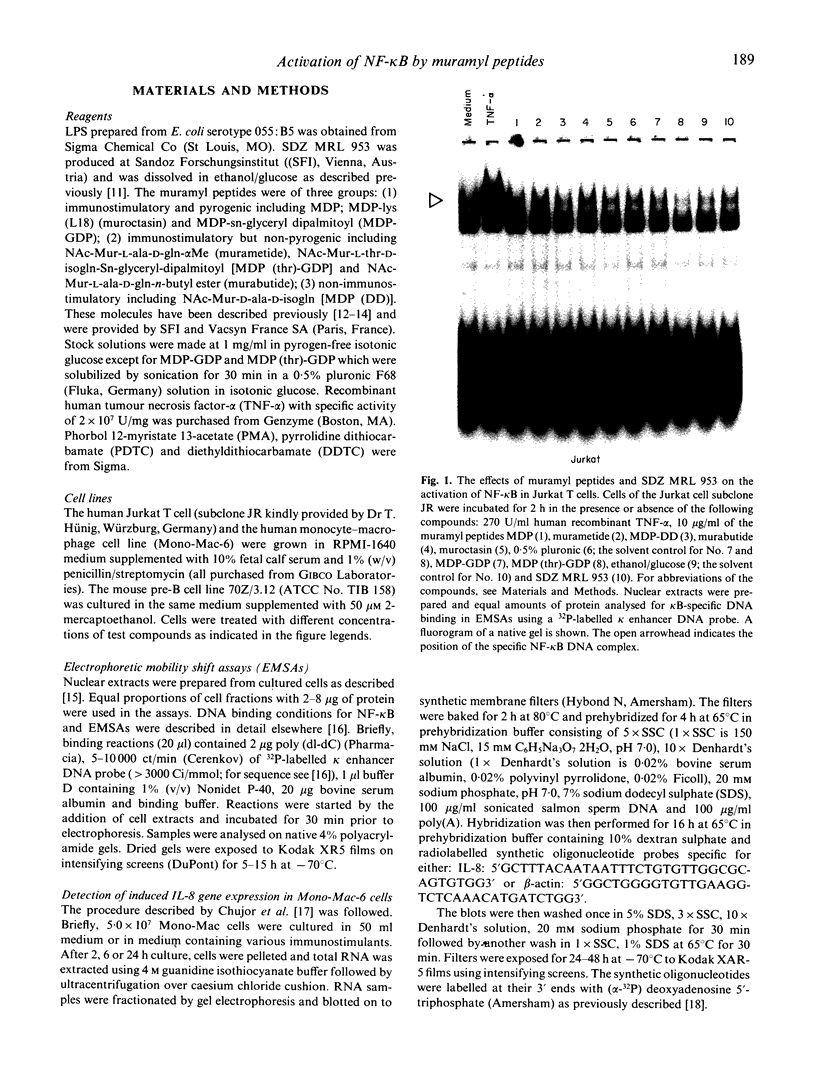
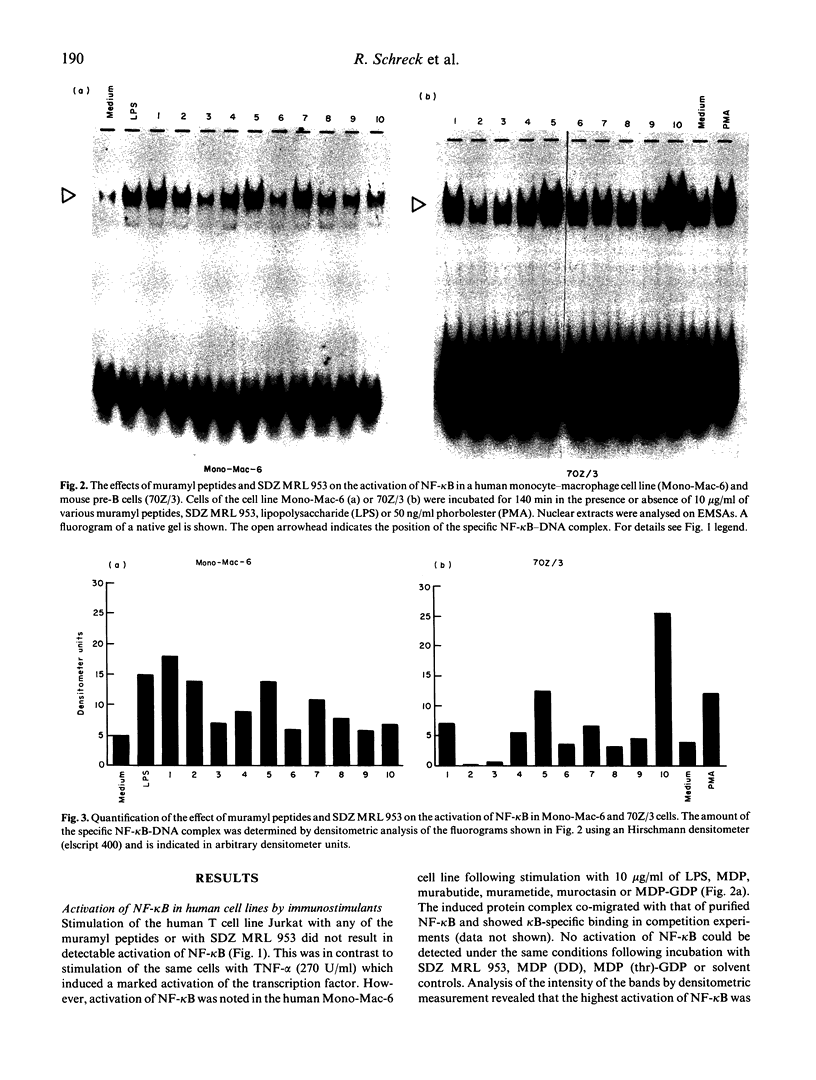
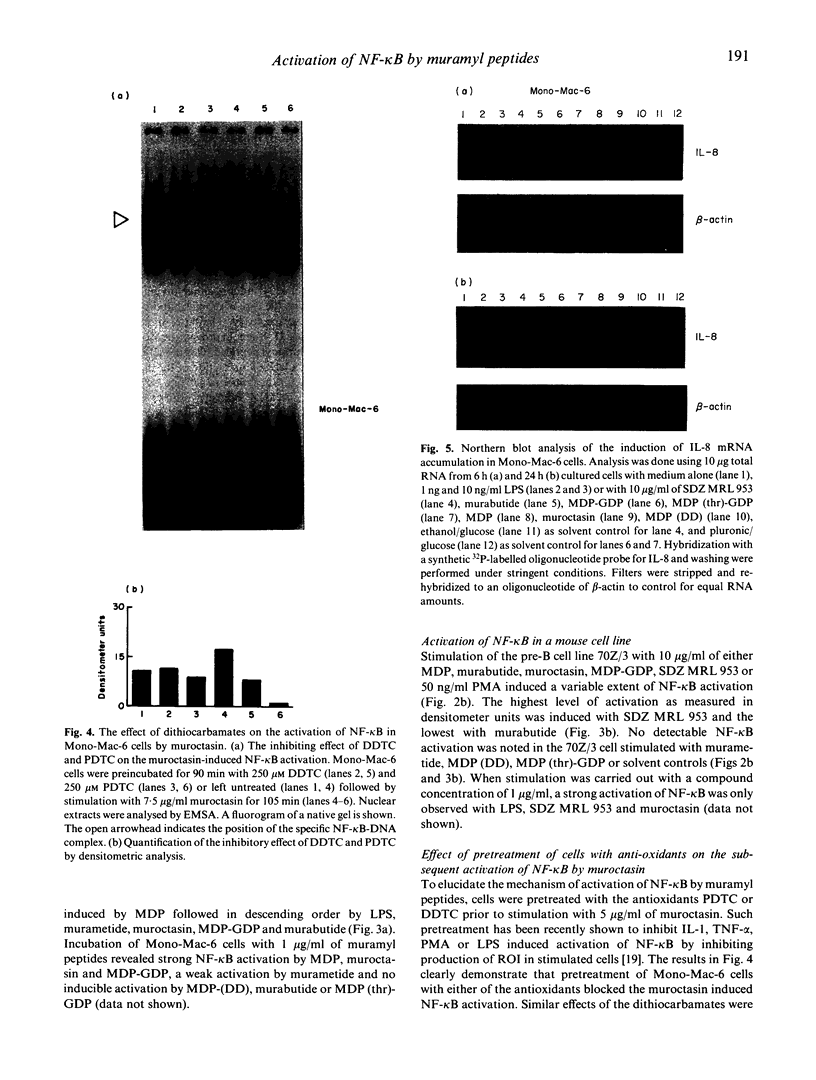
Activation of the cellular transcription factor nuclear factor-kappa B (NF-kappa B) by cytokines and other immunostimulants has been tightly linked with enhanced replication of human immunodeficiency virus-type 1 (HIV-1) in infected cells. Various immunomodulators are currently being examined in animal and human trials for their suitability as adjuvants in potential vaccines against acquired immunodeficiency syndrome (AIDS). It may prove to be beneficial to select adjuvants that do not induce NF-kappa B activation and particularly if the vaccines are to be aimed at seropositive individuals. We have examined a battery of synthetic immunostimulants of the muramyl peptide family for their ability to activate NF-kappa B in human and mouse cell lines. In this report, we demonstrate selective activation of NF-kappa B in different cell lines and by different muramyl peptides possessing immunostimulatory activities. The mechanism of such activation is apparently via production of reactive oxygen intermediates (ROI) since pretreatment of cells with antioxidants blocked subsequent activation of NF-kappa B. However, among all the molecules tested only one lipophilic, non-pyrogenic adjuvant active muramyl peptide showed a complete lack of NF-kappa B activation in all cell lines tested. This molecule could well become the adjuvant of choice in future AIDS vaccines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrignani S., Montagna D., Jeannet M., Wintsch J., Haigwood N. L., Shuster J. R., Steimer K. S., Cruchaud A., Staehelin T. Priming of CD4+ T cells specific for conserved regions of human immunodeficiency virus glycoprotein gp120 in humans immunized with a recombinant envelope protein. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6136–6140. doi: 10.1073/pnas.87.16.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Bahr G. M., Chedid L. Immunological activities of muramyl peptides. Fed Proc. 1986 Oct;45(11):2541–2544. [PubMed] [Google Scholar]
- Chujor C. S., Kuhn B., Schwerer B., Bernheimer H., Levis W. R., Bevec D. Specific inhibition of mRNA accumulation for lymphokines in human T cell line Jurkat by mycobacterial lipoarabinomannan antigen. Clin Exp Immunol. 1992 Mar;87(3):398–403. doi: 10.1111/j.1365-2249.1992.tb03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Wyand M. S., Kodama T., Ringler D. J., Arthur L. O., Sehgal P. K., Letvin N. L., King N. W., Daniel M. D. Vaccine protection against simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6353–6357. doi: 10.1073/pnas.86.16.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Enhancement of B-cell stimulation by muramyl dipeptide through a mechanism not involving interleukin 1 or increased Ca2+ mobilization or protein kinase C activation. Cell Immunol. 1988 Jan;111(1):10–27. doi: 10.1016/0008-8749(88)90047-0. [DOI] [PubMed] [Google Scholar]
- Edelman R., Tacket C. O. Adjuvants. Int Rev Immunol. 1990;7(1):51–66. doi: 10.3109/08830189009061764. [DOI] [PubMed] [Google Scholar]
- Fahey J. L., Schooley R. Status of immune-based therapies in HIV infection and AIDS. Clin Exp Immunol. 1992 Apr;88(1):1–5. doi: 10.1111/j.1365-2249.1992.tb03029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Kieny M. P., Pinter A., Barre-Sinoussi F., Nara P., Kolbe H., Kusumi K., Chaput A., Reinhart T., Muchmore E. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G. E., Leung K., Folks T. M., Kunkel S., Nabel G. J. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature. 1989 May 4;339(6219):70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Negishi T., Nishimura C. Enhancement of non-specific resistance to viral infection by muramyldipeptide and its analogs. Antiviral Res. 1985 Aug;5(4):207–215. doi: 10.1016/0166-3542(85)90025-7. [DOI] [PubMed] [Google Scholar]
- Jupin C., Parant M., Chedid L. Effect of muramyl peptides and tumor necrosis factor on oxidative responses of human blood phagocytes. Immunol Lett. 1989 Sep;22(3):187–192. doi: 10.1016/0165-2478(89)90188-0. [DOI] [PubMed] [Google Scholar]
- Kaku M., Yagawa K., Nagao S., Tanaka A. Enhanced superoxide anion release from phagocytes by muramyl dipeptide or lipopolysaccharide. Infect Immun. 1983 Feb;39(2):559–564. doi: 10.1128/iai.39.2.559-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Tsujimoto M., Koga T., Nagao S., Tanaka A., Kawata S. Chemical structure and biological activity relationship of bacterial cell walls and muramyl peptides. Fed Proc. 1986 Oct;45(11):2534–2540. [PubMed] [Google Scholar]
- Krueger J. M., Walter J., Karnovsky M. L., Chedid L., Choay J. P., Lefrancier P., Lederer E. Muramyl peptides. Variation of somnogenic activity with structure. J Exp Med. 1984 Jan 1;159(1):68–76. doi: 10.1084/jem.159.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C., Schütze E., Hildebrandt J., Aschauer H., Liehl E., Macher I., Stütz P. SDZ MRL 953, a novel immunostimulatory monosaccharidic lipid A analog with an improved therapeutic window in experimental sepsis. Antimicrob Agents Chemother. 1991 Mar;35(3):500–505. doi: 10.1128/aac.35.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancier P., Derrien M., Jamet X., Choay J., Lederer E., Audibert F., Parant M., Parant F., Chedid L. Apyrogenic, adjuvant-active N-acetylmuramyl-dipeptides. J Med Chem. 1982 Jan;25(1):87–90. doi: 10.1021/jm00343a018. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Davison-Fairburn B., Montelaro R. C., Miller M., West M., Ohkawa S., Baskin G. B., Zhang J. Y., Putney S. D. A formalin-inactivated whole SIV vaccine confers protection in macaques. Science. 1989 Dec 8;246(4935):1293–1297. doi: 10.1126/science.2555923. [DOI] [PubMed] [Google Scholar]
- Nabel G. J. HIV. Tampering with transcription. Nature. 1991 Apr 25;350(6320):658–658. doi: 10.1038/350658a0. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T., Une T., Osada Y. Stimulation of non-specific resistance to infection by muroctasin. Arzneimittelforschung. 1988 Jul;38(7A):969–976. [PubMed] [Google Scholar]
- Pomerantz R. J., Feinberg M. B., Trono D., Baltimore D. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human immunodeficiency virus type 1 expression. J Exp Med. 1990 Jul 1;172(1):253–261. doi: 10.1084/jem.172.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk J. Prospects for the control of AIDS by immunizing seropositive individuals. Nature. 1987 Jun 11;327(6122):473–476. doi: 10.1038/327473a0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador L., Burke R. L., Ott G., Van Nest G. The effect of adjuvants on the efficacy of a recombinant herpes simplex virus glycoprotein vaccine. J Immunol. 1988 Sep 1;141(5):1720–1727. [PubMed] [Google Scholar]
- Schild G. C., Minor P. D. Modern vaccines. Human immunodeficiency virus and AIDS: challenges and progress. Lancet. 1990 May 5;335(8697):1081–1084. doi: 10.1016/0140-6736(90)92645-x. [DOI] [PubMed] [Google Scholar]
- Schmid R. M., Perkins N. D., Duckett C. S., Andrews P. C., Nabel G. J. Cloning of an NF-kappa B subunit which stimulates HIV transcription in synergy with p65. Nature. 1991 Aug 22;352(6337):733–736. doi: 10.1038/352733a0. [DOI] [PubMed] [Google Scholar]
- Schreck R., Meier B., Männel D. N., Dröge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992 May 1;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Chedid L. A. Future prospects for vaccine adjuvants. Crit Rev Immunol. 1988;8(2):83–101. [PubMed] [Google Scholar]
- Watson J., Whitlock C. Effect of a synthetic adjuvant on the induction of primary immune responses in T cell-depleted spleen cultures. J Immunol. 1978 Jul;121(1):383–389. [PubMed] [Google Scholar]
- Zabel U., Schreck R., Baeuerle P. A. DNA binding of purified transcription factor NF-kappa B. Affinity, specificity, Zn2+ dependence, and differential half-site recognition. J Biol Chem. 1991 Jan 5;266(1):252–260. [PubMed] [Google Scholar]